|
Find the latest updates on all areas of the ORC, including the IACUC, the NTR IRB, RCOI, and International Compliance.
This quarter we introduce our ORC member – Stacy Abraham!
In this issue, we’ll help you get to the “Yes” with export control regulations and your International Travel.
In this issue, we answer questions : What do you need to consider/do as a student doing research outside of HSC?
In this special article, we provide Guidance and Best Practices for Ensuring Your Submission Compiles with Research Compliance Regulations and Processes.
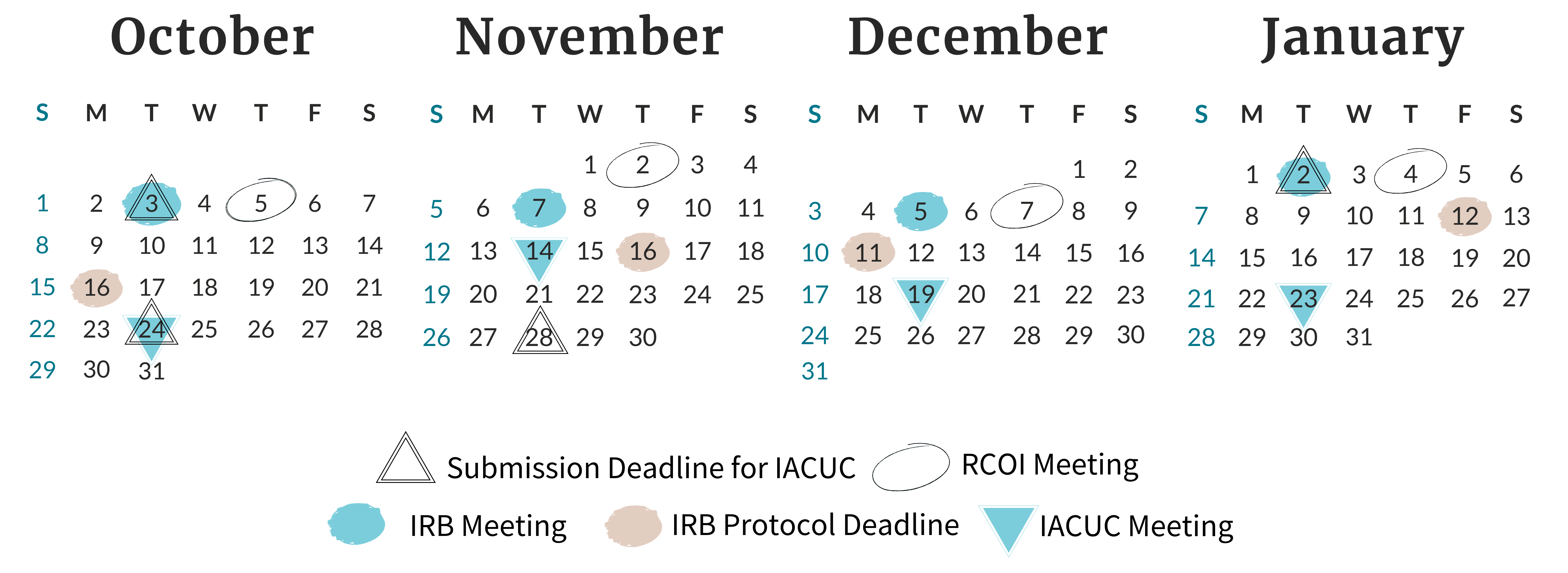
Find all of the meeting and deadline dates for the next three months of Board and Committee meetings.
|
|
What’s Happening in the ORC?
Here is the latest news from the ORC:
- We would like to welcome Jeanne Priddy as our office’s new IACUC Administrator! Ms. Priddy began her new role in the ORC/IACUC in July. Her previous experience at HSC includes working first in DLAM (beginning in 2020) then in Dr. Forster and Dr. Gatch’s lab (in 2021). We are excited to now have Ms. Priddy as part of our ORC team!
- On July 12th, the ORC’s Executive Director, Tania Ghani collaborated with colleagues from UNT Denton’s Research Integrity & Compliance office to co-host the session “What’s An RCOI Committee To Do? Ensuring Appropriate Committee Review and Monitoring of Conflicts of Interest in Research” at UT Dallas’ annual Research Integrity Conference.
- The IACUC Office has a new tutorial video for researchers! This 20-minute video, titled IACUC Tutorial Video: Protocol Submission in GRAMS provides guidance on how to create and submit a protocol utilizing our electronic submission system, GRAMS.
- Meeting dates for Fiscal Year 2024 have been posted for both the NTR IRB and the IACUC.
- The annual Research COI Disclosure Campaign for FY23 wrapped up on October 1st! If you’re a researcher at HSC and you did not complete this disclosure process, please email Research.Compliance@unthsc.edu so that we can work together to ensure you are in compliance with our institutional policies and the federal regulations.
back to top
Employee Spotlight – Meet Stacy Abraham 
How long have you worked in the HSC Office of Research Compliance? What is your role, and what do you like best about it? I joined the Office of Research Compliance in January of 2021. Since 2010 I have worked in research as a Project Manager and educator. It was only a matter of time before I found my spot on this side of research administration!
My role is an IRB Compliance Manager, and my favorite part of the job is learning about new initiatives and getting researchers to the finish line so they can begin their research.
Of the following values, which one do you believe you exemplify the most, and why?(Courageous Integrity, Be Curious, We Care, Better Together, and Show Your Fire!) Better Together! Better Together: I love how well our team works together to tackle all of the challenges that come our way each day. We regularly problem solve and tackle issues together as a team so that we can share our experiences and leverage each of our strengths to provide not only a solution to the immediate problem, but also to create future solutions and guidance. I love my IRB team and couldn’t imagine working anywhere else!
What is something we would be surprised to find out about you? Something you may not know about me is that I enjoy organizing and bring order to chaos. I love to purge! If I was not working as an IRB Compliance Manager, I would love to own my own organization company to help overwhelmed families declutter and make their homes a place of peace and order.
back to top
How to Get to the Yes
International travel and Export Control Regulations
The holiday season has finally arrived, and with it comes exciting opportunities for international travel. You may be traveling to an exotic location to attend a conference. Or you’re simply joining loved ones in a heartwarming family reunion. Either way, you’re excited to temporarily depart from your usual campus routine to take on a taste for adventure. But remember, if you’re packing your HSC-issued laptop alongside your sunscreen or mittens, ensure it’s travel-ready and compliant. Below are some tips and best practices to help you with this: |
|
|
Review and Prepare: To ensure compliance with the United States Export Control Regulations and HSC policies/procedures when traveling internationally (and yes, this includes our friendly neighbors up north in Canada), please follow these steps:
- Visit our official International Travel webpage for information and guidance.
- Complete the International Device Checklist for-export control compliance and screening.
- Upload the checklist to Concur or email it to the Export Control Officer.
Your Destination Matters: Please check if the country you intend to travel to is on the U.S. Department of Commerce’s list of embargoed countries with export controls. If it is, contact the Export Control Officer for tailored advice. Each country has unique laws and regulations for technology and encryption; therefore, the guidance you need may vary. You can find listings of embargoed countries on pages 6-8, in groups D:5 and E.
Consider Alternatives: Don’t worry if your destination has restrictions on encryption technology. HSC’s help desk can provide you with a “clean laptop” that meets all the necessary criteria related to encryption restrictions.
Reach Out for Help: The ORC’s Export Control Officer is here to assist. Please contact her for guidance to ensure your international travels meet all export control requirements at Crystal.Perez@unthsc.edu or Research.Compliance@unthsc.edu.
back to top
|
|
|
You’ve Got Questions, We’ve Got Answers!
Are you a student doing research outside of HSC? Here’s what you need to consider/do!
For students who have been asked to participate in a research project at another institution, this can appear to be a confusing process. But completing research at another institution as an HSC student may be easier than you think with just a few tips:
- Affiliation: Remember! Even if you are working at another institution (which includes being engaged in a research project) you are still an affiliate of the University of North Texas Health Science Center. As such, a friendly reminder that you need to continue to abide by all of HSC’s rules and policies (in addition to the rules/policies at the institution you are working at, of course!).
- Get Guidance from Your Department/Mentor/Advisor! Talk with your mentor/advisor from your department regarding what the focus of your research will be, what methods/resources you will need to carry it out, and what relationship (if any) HSC may have with the other institution regarding the research project. Clarifying all of this will help to ensure you are following all necessary and appropriate federal regulations, as well as University rules/policies regarding research.
- Reach Out to the Office of Research Compliance! To ensure you are on the right path (aka, following all appropriate processes and procedures), contact the ORC. Talking with the appropriate individuals in our office well ahead of time will save you time and energy down the road, by ensuring you are following all appropriate processes and procedures from the start.
- Note for Human Subject Research Projects: You will need to ensure you submit your project to the North Texas Regional IRB for review and approval, prior to starting your project! A reminder that North Texas Regional IRB approval is necessary for ANY research being conducted by NTR IRB affiliated faculty, staff, or students involving human subjects (or identifiable data derived from human subjects) in order to comply with federal regulations and institutional policy. IRB approval cannot be obtained retroactively and must be obtained prior to initiating any research activities with human subjects (including study recruitment).
- Students May Not Serve as PIs: Please note that as an HSC student, you cannot serve as a Principal Investigator (PI) on a research project! Please find a mentor or faculty member to serve as your PI.
- Don’t Forget About Your Conflict of Interest Disclosure! As an HSC student, remember that even if you are involved in research at another institution, you are still involved in research! As such, please remember to complete an appropriate research conflict of interest (RCOI) disclosure form and submit the form to the HSC RCOI office.
You can find additional guidance, tips, and resources here:
HSC Office of Research Compliance Home page
Office of Research Compliance Contacts page
North Texas Regional IRB – IRB Guidance for Students page
HSC Research Conflict of Interest page
We look forward to helping you as you begin or continue on your research journey!
We are here to help you succeed! We hope this section will help you throughout your submission process.
Have a question you would like answered? Just click the link below to submit your question.
back to top
|
|
|
Research Appreciation Day
Guidance and Best Practices for Ensuring Your Submission Complies with Research Compliance Regulations and Processes
It is that time of year again…where we start thinking about Research Appreciation Day (RAD) at HSC. RAD is a wonderful opportunity for researchers to share their research projects, initiatives and efforts to the HSC and wider, general community. RAD is currently scheduled for March 25-28, 2024.
As you consider your RAD submission, HSC Office of Research Compliance offers some helpful tips and reminders for research projects that are subject to the federal regulations related to animal and human subject/participant research. Please see below for specifics.
For Animal research related projects:
If your RAD project includes the use of live animals in the study (in vivo), then IACUC approval is required, regardless of where the animal work is being or was performed.
For Animal studies conducted at HSC, the HSC IACUC will need to review and approve the animal study before one can engage in research involving animal activities. This can be done either through a new IACUC Protocol or an amendment to an existing IACUC Protocol. If submitting a new protocol, please take into consideration the Protocol Submission Deadlines and Meeting Dates found on the IACUC website. The HSC IACUC Protocol Number will need to be provided in the RAD Submission, along with a copy of the IACUC Protocol Approval Letter.
For Animal studies conducted at a different performance site, you will need to provide a copy of the IACUC protocol approval letter from the IACUC that reviewed and approved the animal study. This will show the animal study was conducted on an approved IACUC Protocol.
For Animal studies performed solely on animal tissues (in vitro studies), while there is no need to have IACUC approval, there is a need to have IBC approval. The RAD application will ask for the source of the animal tissue. This does not refer to the species of animal, nor the specific tissue used, but rather from where the tissue was acquired. If from a live animal, an IACUC protocol number would suffice for the source. However, if animal tissue was purchased from a vendor, then provide the name of the vendor of where the tissue was purchased.
Should you have any IACUC Specific questions regarding your RAD Submission, feel free to contact the IACUC Office at IACUC@unthsc.edu.
For Human Subject/Participant research related projects:
Research projects involving human research participants, as defined by the regulations, require appropriate Institutional Review Board (IRB) review and determination or approval prior to initiating any research activities.
Per institutional procedures, the North Texas Regional IRB (or NTR IRB) makes this determination and, where appropriate, has appropriate oversight of human subject/participant research conducted by its affiliates (employees, faculty/staff and students from HSC, John Peter Smith Hospital Network, UNT Dallas and Tarrant County Public Health), independent of where the research is being conducted.
Tips for a successful IRB submission of your RAD related research project:
- Do not wait until the last minute! Be mindful of submission deadlines (IRB or RAD driven) and build in appropriate time and flexibility to meet those deadlines and proposed objectives. (Please note that RAD is a separate submission process from that of the IRB).
- For example, if you need to collect data to present at RAD, IRB review/determination of your research project must be obtained well in advance to allow for the IRB review/approval and data collection processes. Remember, you cannot start data collection until the IRB approves your project. Note that IRB review times are subject to staff availability and current workload. While the NTR IRB will do its best to meet desired deadlines/timelines, we may not always be able to accommodate quick turnaround times. Please advise the NTR IRB of any required or desired timelines/deadlines upon submission. Bottom line, it is never too early to submit!
- Please use the available IRB resources/guidance materials to facilitate your submission.
- Guidance for RAD participants – Provides more tips and submission guidance/instruction for specific types of research projects typically submitted for RAD (chart reviews, surveys, and secondary data analyses)
- New Project Consultation Form – Set up a time to meet with an IRB representative to discuss your project!
- Explore the NTR IRB website for more guidance on submission procedures, research projects, etc.
- Have IRB-related questions? Contact us at NorthTexRegIRB@unthsc.edu
- Ensure you have sufficient documentation, evidence, and information to demonstrate that appropriate IRB review/determination of your research project was obtained. This includes the IRB determination/approval letter and the IRB number associated with your RAD project/abstract.
- For projects already approved by the IRB, please ensure individuals listed on the RAD abstract have received appropriate IRB approval or authorization for their engagement, if any, in research activities.
For specifics on RAD submission deadlines, questions related to the RAD submission process, or other RAD-related questions, please visit the RAD website.
back to top
|



Social media