Research Compliance Newsletter: Quarter Two, June 2022
June 1, 2022 • Research Compliance

What’s Happening in the ORC?Find the latest updates on all areas of the ORC, including the IACUC, the NTR IRB, RCOI, and International Compliance. Employee Spotlight – Meet Amanda!This quarter we introduce our team member – Amanda Oglesby! How to Get to the “Yes”In this issue, we’ll help you get to the “Yes” with our overview of the Grants & Research Administration Management Suite (GRAMS) You’ve Got Questions, We’ve Got Answers!In this issue, we answer questions about submitting a closure request, when a new protocol should be submitted for the IACUC, and what is considered Protected Health Information (or PHI). International Compliance – An OverviewIn this special article, we provide a brief explanation of what International Compliance is and what it means for researchers. ORC CalendarFind all of the meeting and deadline dates for the next three months of Board and Committee meetings.
|
|
What’s Happening in the ORC?There have been a few staffing changes in the ORC since the last quarter!
In addition to recent staffing changes, the International Compliance program has been transitioned into our department. You can learn more about International Compliance in this quarter’s article, International Compliance – An Overview.
Our office has been working diligently on updating current/creating new procedures, webpages, and guidance materials in order to better serve the research community: RCOI:With the integration of the new Conflict of Interest (COI) module, RCOI disclosures will now be submitted and reviewed through the Grants & Research Administration Management Suite (GRAMS). To learn more about the COI/RCOI module, please complete the Learn HSC / Bridge Training at GRAMS Conflict of Interest Guide. NTR IRB:The NTR IRB staff have updated the “Protocol Synopsis Template (General)” document. Guidance for filling out the general protocol synopsis template is available on the “Master Protocol Synopsis Guidance Sheet”. Investigators should use the updated, general protocol synopsis template for all new human subject research projects, with the exception of chart review studies. A separate protocol synopsis template, the Chart Review Protocol Synopsis Template, is available for studies that involve only chart reviews. IACUC:As part of the ongoing Process Improvement initiative, the IACUC Office recently implemented a new process for approving laboratories for animal use. The IACUC Office created a web page, called the Laboratory Approval Process for Animal Use, to clarify this new process. Adding a new laboratory space for animal use requires an inspection, especially for spaces not previously approved for animal use. This web page provides details for researchers to request, as well as to prepare for, this inspection. As always, researchers are welcome to contact the IACUC Office with any questions in regard to approving new laboratory spaces for animal use.
Curious about how you can keep up with updates happening in both the NTR IRB and the IACUC? You can find all past announcements in their respective Announcement Archives: the IRB Announcement Archive and the IACUC Announcement Archive. Employee Spotlight – Meet Amanda Oglesby |
How to Get to the YesAn Overview of the Grants & Research Administration Management Suite (GRAMS) We are so excited that our new electronic research administration/compliance system is finally here! As many of you may know, the UNT System (HSC, UNT Denton, and UNT Dallas) launched “GRAMS” (a Huron product) in September 2021. The first module introduced was the IACUC, followed a few months later by the Grants & Agreements module. In May 2022, the latest module in the suite, Conflict of Interest (COI, which includes both Institutional COI as well as Research COI) debuted; the IRB module will be forthcoming within the next year (specific implementation and “go-live” dates are still pending).
GRAMS allows our researchers a single portal which integrates the functions of all of the above areas (e.g., IACUC, Grants & Agreements, COI, IRB, etc.) and improves routing and business processes for more efficient research submissions. Other modules in this research administration suite may be launching in the future – so stay tuned!
All UNT System affiliates may access GRAMS through a single sign-on using your UNT System username and password.
For additional information regarding the GRAMS system, including information about training regarding using the system as well as an overall timeline, please visit: https://www.unthsc.edu/research/grams/.
And of course, if you have any questions at all about using the system, do not hesitate to reach out to our office at 817-735-0409! We are always happy to help! 😊 |
||
You’ve Got Questions, We’ve Got Answers!1. Q:Do I need to submit a closure request for my protocol once I’m done with it?A: Yes! For specific guidance regarding submitting a closure request for either an IRB protocol or an IACUC protocol, please see below:
2. Q: When do I need a new IACUC protocol? (i.e., I have a new grant – do I need a new protocol for it?)A: An IACUC protocol needs to be submitted if you will be conducting research, teaching or testing in which you will need to use live animals. A new IACUC protocol will need to be submitted with the addition of a new grant proposal if any of the current goals of the research project deviate from the current protocol goals. In addition, regulations require a de novo review of previously approved ongoing protocols prior to the end of the third year. UNTHSC IACUC fulfills this requirement by requiring a new protocol to be submitted for the project before the expiration date in its third year. Please note that if the protocol is not resolved before the expiration date, you may need to stop all animal work until your protocol is approved. 3. Q: What is considered protected health information (PHI)?A: Per HIPAA Privacy Rules, protected health information is individually identifiable health information, including demographic information, which relates to:
The 18 HIPAA Identifiers include: 1. Names; 2. All geographical subdivisions smaller than a State, including street address, city, county, precinct, zip code, and their equivalent geocodes, except for the initial three digits of a zip code, if according to the current publicly available data from the Bureau of the Census: (1) The geographic unit formed by combining all zip codes with the same three initial digits contains more than 20,000 people; and (2) The initial three digits of a zip code for all such geographic units containing 20,000 or fewer people is changed to 000. 3. All elements of dates (except year) for dates directly related to an individual, including birth date, admission date, discharge date, date of death; and all ages over 89 and all elements of dates (including year) indicative of such age, except that such ages and elements may be aggregated into a single category of age 90 or older; 4. Phone numbers; 5. Fax numbers; 6. Electronic mail addresses; 7. Social Security numbers; 8. Medical record numbers; 9. Health plan beneficiary numbers; 10. Account numbers; 11. Certificate/license numbers; 12. Vehicle identifiers and serial numbers, including license plate numbers; 13. Device identifiers and serial numbers; 14. Web Universal Resource Locators (URLs); 15. Internet Protocol (IP) address numbers; 16. Biometric identifiers, including finger and voice prints; 17. Full face photographic images and any comparable images; and 18. Any other unique identifying number, characteristic, or code (note this does not mean the unique code assigned by the investigator to code the data). For research purposes, if the dataset includes health information about an individual in addition to one of the 18 identifiers listed above, that dataset would be subject to HIPAA Privacy Rules and certain considerations need to be met including ensuring data storage and confidentiality procedures are HIPAA compliant. We advise researchers to reach out to ITSS (informationsecurity@unthsc.edu) to confirm the data platform/storage system being used will meet all compliance regulations for their particular study. Please note that HIPAA regulations state that PHI should be collected at the minimum necessary standard for research purposes. Essentially, only those variables/PHI that are absolutely necessary to conduct the research should be collected/maintained as part of the research data. Please bear this in mind when designing your research. We are here to help you succeed! We hope this section will help you throughout your submission process. Have a question you would like answered? Just click the link below to submit your question. |
||
International Compliance – An OverviewAs you may (or may not) have heard, the HSC Office of Research Compliance officially took over the HSC International Compliance program in March 2022. But…what is “International Compliance” you ask? Well, great question! Here is a quick overview to explain, “But what does it all mean???”
International Compliance (aka, Export Controls) “includes the shipment or transfer of equipment, articles, services, or encryption software to another country as well as the transfer of technical data or information to a foreign national, whether it occurs in the U.S. or abroad.” So, essentially, this could include, but not be limited to, the following areas: research projects which involve transferring data/information overseas; working with foreign nationals (i.e., individuals who are not US citizens or, regardless of citizenship, are representing a foreign government, foreign corporation, or other foreign entity); traveling outside of the U.S. for an HSC-sponsored reason/event (e.g., travel to another country is necessary for the purposes of an HSC grant or a conference which is related to an HSC project you are working on, etc.).
As you can guess, HSC (and all its affiliates, such as faculty, researchers, staff, students) must fully abide by certain federal laws and regulations regarding International Compliance, which include the following:
UNTHSC research activities may remain compliant with international compliance/export control laws under the fundamental research exclusion by ensuring that it meets the definition of fundamental research: “…research in science, engineering, or mathematics, the results of which ordinarily are published and shared broadly within the research community, and for which the researchers have not accepted restrictions for proprietary or national security reasons.”
PLEASE BE REMINDED that International Compliance applies to all activities which include export controls and sponsored research projects. Violation of export control laws and regulations can result in significant civil and criminal penalties for the Health Science Center and for the individual researchers involved. Interested to learn more?Check out the official HSC International Compliance Policy (2.106) and visit the ORC International Compliance – Export Controls website. Still have questions?Don’t hesitate to reach out at Research.Compliance@unthsc.edu or call the ORC main line (x0409). |
||
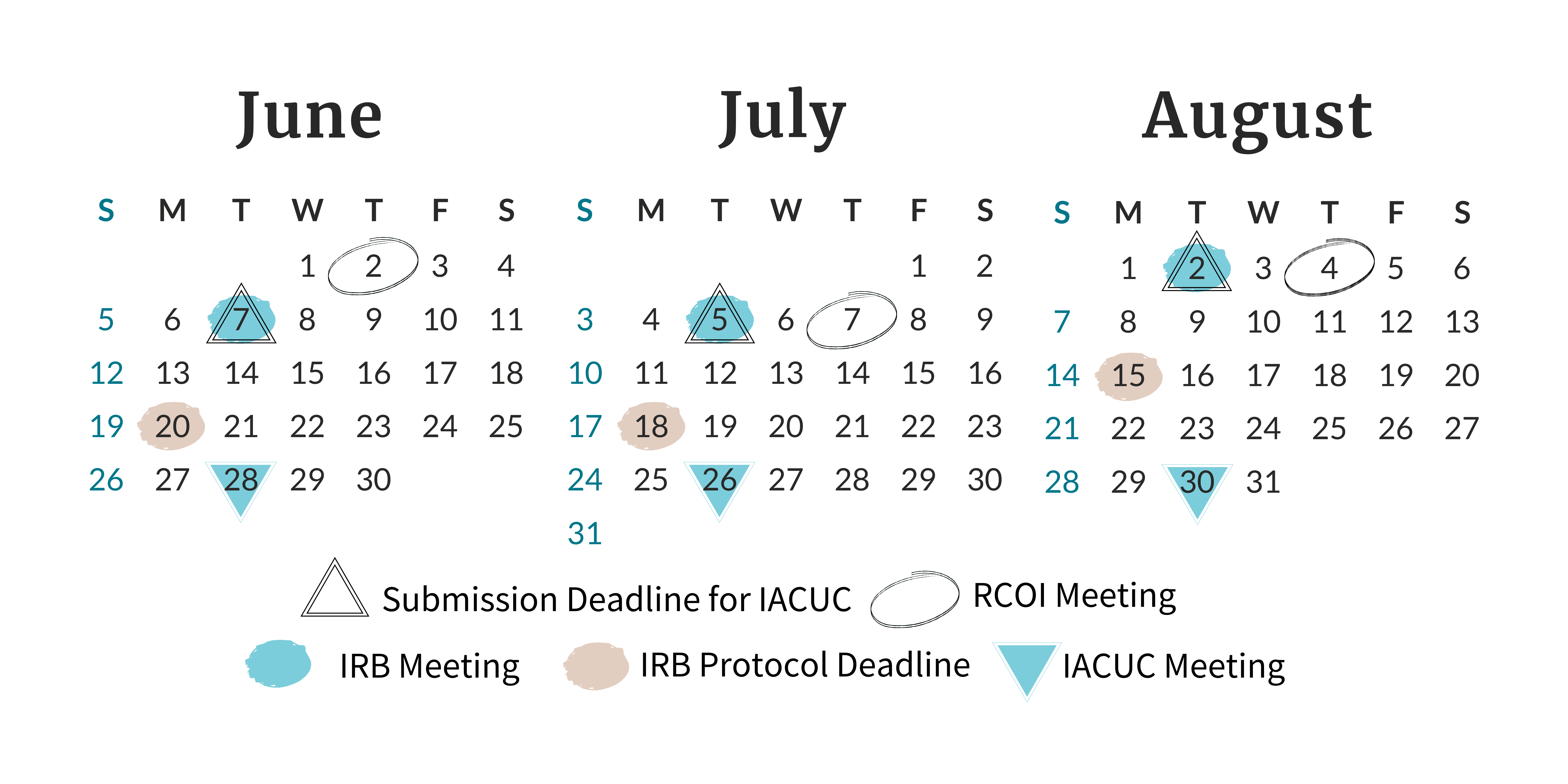
ORC Calendar
|


Social media