|
Find the latest updates on all areas of the ORC, including the IACUC, the NTR IRB, RCOI, and International Compliance.
This quarter we Spotlight our whole team by asking everyone about their favorite holiday tradition!
In this quarter’s issue, we’ll help you get to the “Yes” with our tips on training requirements.
In this issue, we answer questions about getting started as a new researcher at HSC and affiliated institutions. If you’re a new researcher, this Q&A is for you!
In this special article, we got the scoop on Dr. Cunningham’s research, his experience working with our office, and the tips and tricks he has for maintaining compliance.
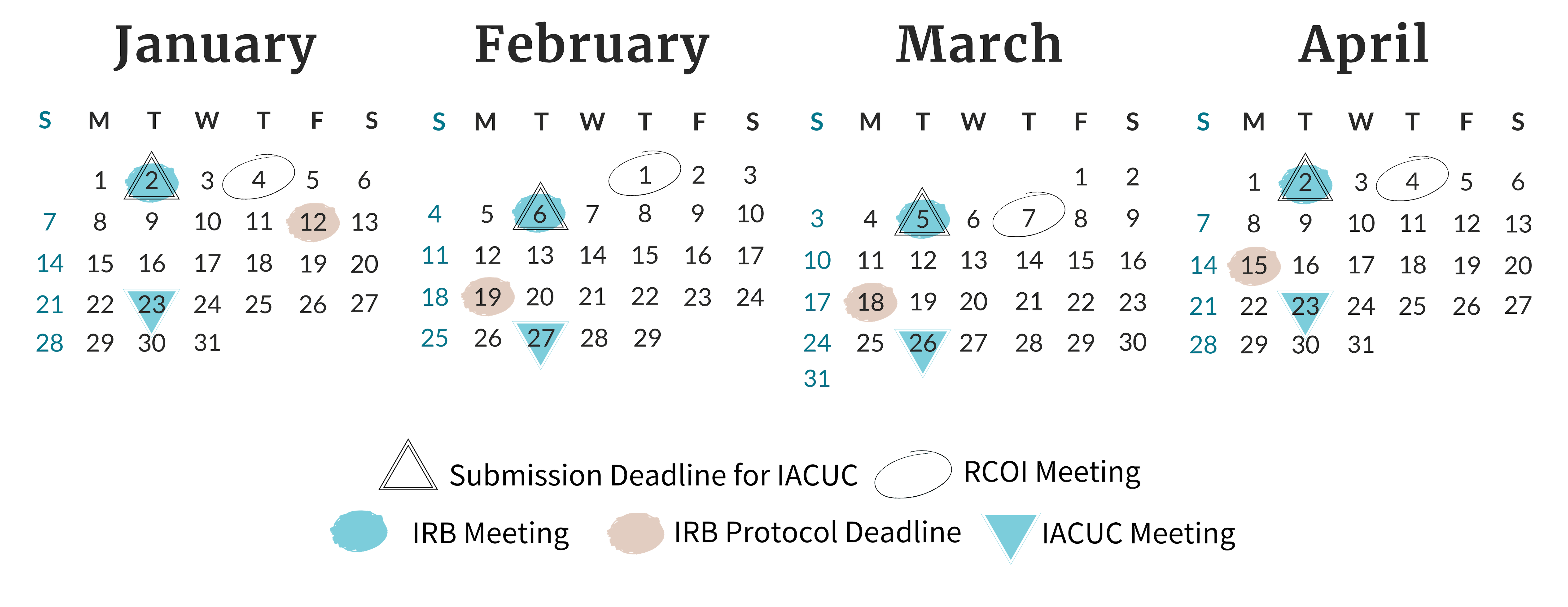
Find all of the meeting and deadline dates for the next three months of Board and Committee meetings.
See if you can find all the Research Compliance related terms and concepts in the special edition word search!
|
|
What’s Happening in the ORC?
Happy New Year! We hope everyone had a fun and safe holiday season! For the last quarter of Calendar Year 2023, we bring you a special “Best Of” edition of our ORC Newsletter where we bring back some of our favorite articles from the last two years.
Although these articles have been shortened here, you can click the title of each to view the original version.
Like the rest of 2023, the last quarter remained a busy time for our office:
- At the beginning of December, our IACUC Assistant Director, Christina Aguilar, co-presented two sessions at the 2023 Public Responsibility in Medicine and Research (PRIM&R) Annual Conference: “The Invisible Role of IACUC Administrators” and “Review Cycles for Documents (SOPs, Protocols, Policies): How Best to Schedule”.
- Several of our staff also attended the PRIM&R Annual Conference, and gained a plethora of knowledge in regard to both animal and human subject research compliance practices. Our team is excited to begin implementing some of these new ideas into our procedures and guidance documents – stay tuned!
- The North Texas Regional IRB also spent a good majority of last year preparing the Step One application for The Association for the Accreditation of Human Research Protection Programs, Inc. (AAHRPP), the accrediting body for HRPPs. The Step One application was officially submitted at the end of December. During this process, we created or updated several IRB documents and resources, including the following:
Submission of the Step One application is just the first formal step in our road to accreditation of our human subject research protection program – we will provide further updates as this process continues.
A friendly reminder that Research Appreciation Day is coming up the week of March 21st – 28th! Submissions opened earlier this month, and close in February. For more information, click here.
back to top
“Best Of” Edition – Meet the Team!
For this “Best Of” edition, we decided to Spotlight our entire department instead of just one employee! We asked everyone what their favorite holiday tradition is, see their answers below!

Jeanne Priddy, BS (IACUC Administrator): My favorite holiday tradition is watching The Thin Man on Christmas Eve with my husband. The Thin Man is a 1934 comedy/mystery film set around Christmas and the film’s protagonists, Nick and Nora Charles, are my favorite on-screen couple and it is one of my favorite movies. It just doesn’t feel right to watch it any other time of the year.
Joycelyn Bryant (Senior Administrative Coordinator, Research Compliance): We have had various traditions over the years with my immediate family and extended family. One of the recent traditions that we have incorporated is that we forgo buying Christmas gifts for ourselves bi-annually and instead spend that money to buy gifts for families in need. Additionally, we volunteer as a family to feed the homeless or spend time with the elderly residents in local nursing homes.
Ellie Knutson, MA (Education and Outreach Specialist, Research Compliance): Although we have several extended family traditions, my favorite is one with just me and my husband. For Valentine’s Day, we avoid the crowds by staying in and watching the 1993 crime/romance movie, True Romance.
Alyson Stearns, CIP (IRB Compliance Manager): My favorite tradition during the holiday season is making my special peppermint hot cocoa for my family. My daughter loves it and asks for it daily! It’s even better when we sip it next to a warm fire in our fireplace.
Stacy Abraham, MPH (IRB Compliance Manager): My favorite holiday tradition is egging our friends’ yards for Easter! Each year we fill eggs with treats and place them around the yard of our friends with young children so they can have their own surprise easter egg hunt. We always leave one empty to remind them that Jesus has Risen!
Christina Aguilar, CPIA (Assistant Director, IACUC): My favorite holiday tradition is celebrating Japanese New Year on January 1st. We start the day with a traditional Japanese New Year breakfast soup, called ozoni. We then enjoy a lunch including lots of Japanese cuisine, including the food I prepared for the Osechi Ryori (traditional Japanese foods associated with the New Year). The day is not complete until I make an origami animal representing the animal for the year according to the Chinese zodiac. It is a fun day spent with friends and family, and a wonderful way to bring in the new year.
Amanda Oglesby, MS, CIP (IRB Compliance Manager): My favorite holiday tradition is participating in a “crafts” day with family and friends. We spend the day creating ornaments and have fun decorating them with paint, markers, and glitter!
Crystal Perez, DPS, CHW, MS, CHWC (Export Controls and Research Ethics Officer, Research Compliance): Our all-time favorite holiday tradition is when we load the whole family into the car for our annual Christmas lights tour around our neighborhood. It’s like stepping into a magical winter wonderland, and it reminds us of the true spirit of the season – togetherness and joy. We wait until another special day to gather around the dinner table for another beloved tradition: sharing and savoring homemade dishes that each one of us brings. For me, it’s the homemade mac and cheese and the sweet potato casserole that steal the show. But what truly warms my heart is being with my family, spending precious time together.
Tania Ghani, MS, CIP (Executive Director, Research Compliance): We have had several holiday traditions throughout my life, but I have to say, my favorite has always been having a nice, traditional Thanksgiving dinner with my family (whoever is available to join!). My father loved Thanksgiving and after he passed away, my brother and I have done our best to try and keep up the tradition of hosting at one of our houses each year. It’s also nice to see the next generation (all of my nieces and nephews) getting into the spirit as well by making special desserts every year. Additionally, my husband and I had started a tradition after we got married to have a relaxing brunch (at a place of our choosing) on New Year’s Eve day, every year. We like to spend that time reflecting on the past year, and planning what we’d like to accomplish in the coming year.
Itzel Pena Perez, MS, CIP (Director, NTR IRB): One of my favorite holiday traditions is celebrating Three Kings Day (or Epiphany) on January 6th. We celebrate the day with more gifts and a “rosca de reyes” (an oval shape Mexican sweet bread decorated with dried fruit and confectionery) with a baby Jesus hidden inside the cake. Those who get the baby Jesus must throw a party (with tamales) on February 2nd! So, our Christmas tree (without any shame) remains up until February 2nd!
back to top
How to Get to the Yes
Training for Researchers
It’s a pretty familiar scene…you’ve prepared the draft of your research protocol and hit the submit button…only to get a comment back that either you and/or members of your study team have not completed (or submitted) the appropriate trainings. Now what?
Fear not! The Office of Research Compliance has outlined the specific trainings you or your research staff need to complete before engaging in research. |
|
|
You’ve Got Questions, We’ve Got Answers!
Q: I am new to research and research compliance here at HSC – what do I do, who do I contact, and how do I get started???
A: The first thing to know is that the ORC is here to help you and aid in the success of your research!
Here are the areas with which the Office of Research Compliance can assist you:
- If you will be conducting animal research – we invite you to reach out to our Institutional Animal Care and Use Committee (IACUC) specialists! To get started, you can review the “New Investigator” page on the IACUC website, or email IACUC@unthsc.edu. You can also request to set-up a consultation with one of our IACUC staff by accessing our IACUC Consult Request Form.
- If you will be conducting human subject research – we invite you to reach out to our North Texas Regional (NTR) IRB crew! The NTR IRB will work with you to ensure that your project is reviewed and approved appropriately (prior to commencing), and that it adheres to all appropriate federal/institutional regulations and policies. A plethora of IRB guidance can be found at our North Texas Regional IRB website; the NTR IRB has also created some introductory guidance videos, entitled “Guidance and Resources” and “IRBNet Tutorial for Researchers”. You can also request a consult with one of our dedicated NTR IRB staff by accessing our “Request a Consultation” form or send any questions via email to NorthTexRegIRB@unthsc.edu.
- International Compliance / Export Controls – if your research will involve transfer of equipment, articles, services, or encryption software to another country, and/or the transfer of technical data or information to a foreign national, we invite you to review information on our International Compliance – Export Controls webpage, or reach out to our Export Controls Officer, Crystal Perez, at Research.Compliance@unthsc.edu.
A quick side note that the ORC/International Compliance office is also responsible for conducting risk assessment reviews for anyone who will be traveling internationally (to countries which are determined to be higher risk) as part of their research or any HSC official/sponsored business. All individuals traveling internationally for HSC official business purposes should review information on the UNT System International Travel page, and also ensure their travel is registered in the Concur system.
- Post-Approval Monitoring – Please note that for all research studies under the purview of the IACUC or NTR IRB are eligible for routine/periodic (or not for-cause) post-approval monitoring audits! Information on the respective animal and human subject post-approval monitoring audit processes can be found via the following links:
- Training – as noted above, there are certain training requirements researchers will need to complete, depending on the type of research. Links for specific training requirements are provided below, however, you are always welcome to reach out to the ORC for any questions regarding training requirements!
Any other general questions or not sure where to start? You can visit our Office of Research Compliance webpage, call us at 817-735-0409, or email us at Research.Compliance@unthsc.edu.
We look forward to hearing from you!! 😊
Have a question you would like answered? Just click the link below to submit your question.
back to top
|
|
|
Interview with Dr. Tom Cunningham: Why It’s Important for Researchers to Work With the ORC

“Nontraditional.” This is how Dr. Tom Cunningham describes his research history background in one word.
He explained how he got his start in experimental psychology, chuckling that he is “old enough that no neuroscience programs existed” when he was in school, ultimately leading him to receive his MS and PhD in Biopsychology. Currently, he conducts research on the central nervous system by using animal models to look at fluid homeostasis, blood pressure regulation, hypertension, and intermittent hypoxemia related to sleep apnea. With his history of working with animal models, he is no stranger to working with research compliance offices in order to receive and maintain approval for his plethora of protocols.
Dr. Cunningham isn’t shy when it comes to sharing his history with the Office of Research Compliance, stating that his experiences have “varied a lot,” and that “there was a time when the relationship between Research Compliance and Investigator was antagonistic. Now, it is much more collaborative and focused on problem solving.” He isn’t alone in having mixed interactions with our office; when asked for his recommendations for researchers when it comes to working with the ORC, his answer is concise: “show up early, be transparent, and communicate.” What does he mean by that? “A lot of times, PIs are so deep in the weeds, they are not letting [Research Compliance] know why [their work] is important.”
So what advice would he give other investigators for maintaining compliance with their studies? He recommends utilizing the services our office offers such as pre-reviews and consultations. Of course, Dr. Cunningham isn’t without his bias: he was instated as the Associate Vice President for Research Administration in September of 2021. If you’re interested in learning more about Dr. Cunningham’s research, you can find out more by going to his Faculty page or his HSC Experts profile.
back to top
|





Social media