|
Find the latest updates on all areas of the ORC, including the IACUC, the NTR IRB and RCOI.
This quarter we introduce our newest member – Karina Garcia!
In this quarter’s issue, we’ll help you get to the “Yes” with our tips on training requirements.
ORC Frequently Asked Questions and our Q&A.
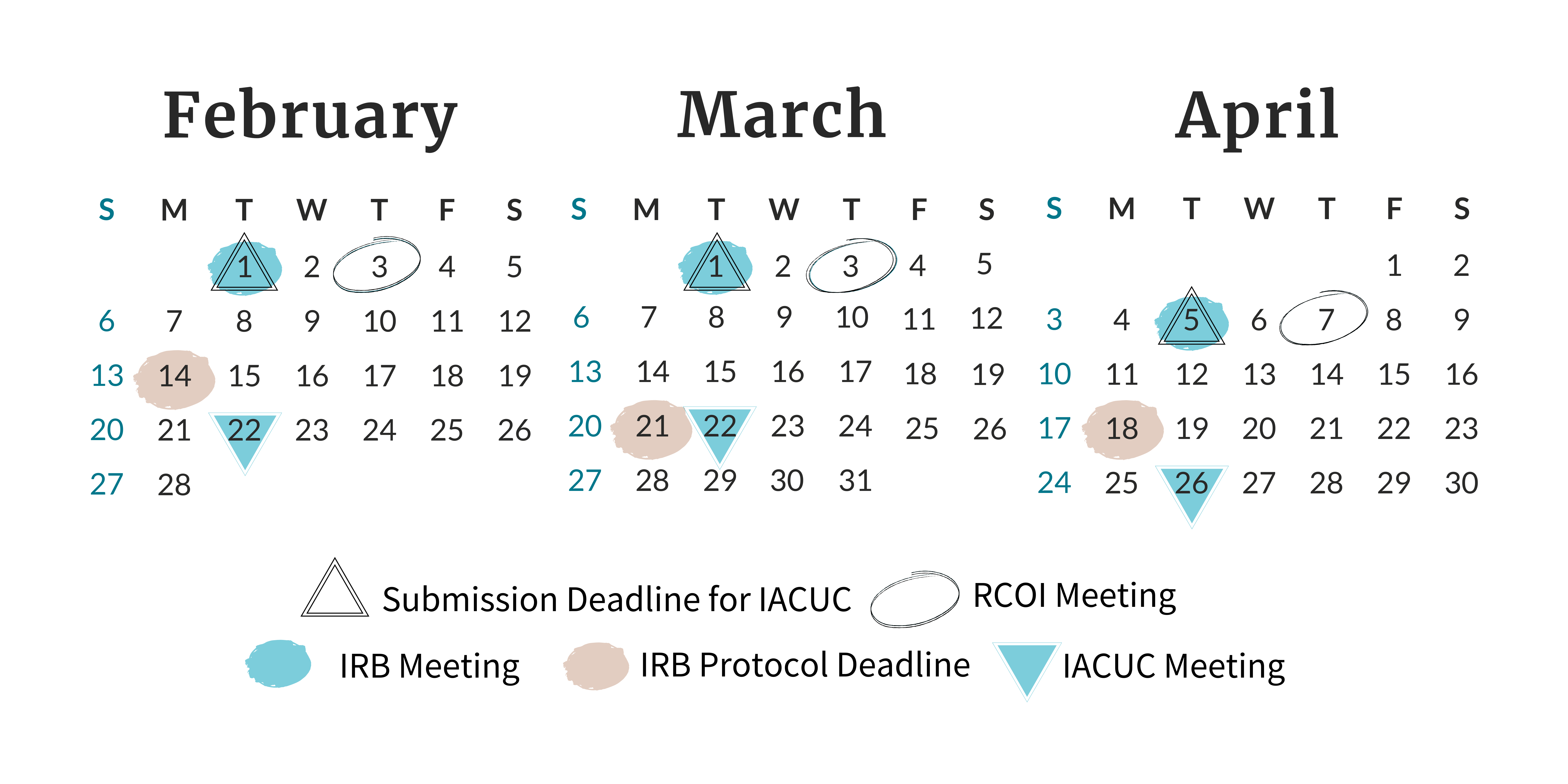
Find all of the meeting and deadline dates for the next three months of Board and Committee meetings.
|
|
What’s Happening in the ORC?
Welcome to the Office of Research Compliance’s first Quarterly Newsletter, where you’ll find updates to our office, tips for getting your protocols approved, get to know our team members, and so much more!
Here are the “Cliffs Notes” of all major updates in our office within the last year:
- We had quite a few staffing changes in 2021 – Stacy Abraham joined the IRB team in January; Anna Laurent moved from her previous role as the IACUC Administrator to her current role as Research Compliance Officer, in March; Karina Garcia started as the IACUC Administrator in June, and sadly, Jessica Bird left the institution due to an out-of-state move in November.
- Our office had a very busy summer! In June, our Research Compliance Officer re-activated audits for Human Subject Research studies as part of our PostApproval Monitoring program. In the same month, the IRB Team completed the first launch in the HSC Process Improvement initiative, and the IACUC Team quickly followed by completing their own Process Improvement launch in July. In addition, the IACUC team launched the IACUC module in the Grants and Research Administration Management Suite (GRAMS) system in September.
- As you may have seen, the IRB and IACUC Process Improvement initiatives have produced MANY changes in the past few months! Several new guidance documents and processes for researchers have been released and more are still to come! So….Stay tuned!
With all of these changes and the positive results they produced, our office is looking forward to seeing what 2022 has in store for the Health Science Center and the ORC!
back to top
Employee Spotlight – Meet Karina Garcia

How long have you worked in the HSC Office of Research Compliance? What is your role, and what do you like best about it? I have worked in the UNTHSC ORC for about 7 months and currently hold the position of IACUC Administrator. My favorite part of this position and the field, is being able to ensure a culture of humane care for research animals, while also encouraging high quality studies that will one day contribute to medical advancements. It is nice to know that my role has a purpose, making it that much easier to look forward to my job duties every day.
Of the five HSC values, which one do you believe you exemplify the most, and why? The UNTHSC value that I exemplify the most is respect, which is also one of my personal values. Growing up my parents instilled this in me at a very young age. To always be respectful, caring and compassionate towards others. It is also how I like to approach my co-workers, and all of my relationships while in the workplace. I carry this value on a personal level, and it is nice knowing that it is also held to a high standard here, at UNTHSC.
What is something we would be surprised to find out about you? I come from a huge family, having a total of 21 aunts and uncles (yikes! I know). So with that comes tons and tons of cousins! Surprisingly, out of all of us not only was I a first- generation college student, but I was also the first to receive a Postgraduate degree.
back to top
|
|
|
How to Get to the Yes
Training for Researchers
It’s a pretty familiar scene…you’ve prepared the draft of your research protocol and hit the submit button…only to get a comment back that either you and/or members of your study team have not completed (or submitted) the appropriate trainings. Now what?
Fear not! The Office of Research Compliance (which includes the UNTHSC IACUC, the North Texas Regional IRB, and Research Conflict of Interest) has outlined the specific trainings you (or your research staff) need to complete before engaging in research (for either Animal Researchers or Human Subject Researchers). Trainings typically include completion of modules in CITI, institutional requirements, and may also include other components, such as Occupational Health and trainings through the Department of Lab Animal Medicine (DLAM; please note these last two areas would be required for animal research only).
General Reminders:
- “CITI” stands for the “Collaborative Institutional Training Initiative” – this is an external organization that provides research, ethics, and compliance trainings. Please note that ORC staff are not able to assist with resolving issues regarding CITI accounts or access – researchers should contact CITI directly for help with these types of issues.
- Individuals should not be added onto a study until they have completed all of their necessary trainings as such, please do not submit a new study or an amendment to an existing study to add personnel until individuals have completed all appropriate trainings. NOTE: It’s important to remain current with the appropriate training(s) (and avoid lapses) while engaged in research.
- All UNTHSC individuals engaged in research are required to complete the Research Conflict of Interest training and disclosure form prior to involvement in a research project, and at least annually thereafter (this is done in conjunction with the Institutional Conflict of Interest disclosure). Additionally, any changes in disclosure status which affect the research project (financial or otherwise) need to be reported within 30 days of the change occurring. Please note that this requirement applies whether the project is funded or unfunded. NOTE: UNTHSC individuals involved in or engaged in research are defined as personnel including, but not limited to, administrators, faculty, staff, post-doctoral fellows, students, interns, residents, collaborators, subcontractors or consultants whose institutional responsibilities include the design, conduct or reporting of research, funded, unfunded or proposed for funding.
For additional information on the specific trainings to complete prior to engagement in a research study, please visit:
For information regarding Research Conflict of Interest, please visit: HSC Research Conflict of Interest
back to top |



Social media