Research Compliance Newsletter: Quarter One, April 2024
April 17, 2024 • Research Compliance

What’s Happening in the ORC?Find the latest updates on all areas of the ORC, including the IACUC, the NTR IRB, RCOI, and International Compliance. Employee Spotlight – Crystal Perez!This quarter we introduce our ORC member – Crystal Perez! How to Get to the “Yes”In this issue, we’ll help you get to the “Yes” by delving into adding a funding source to your IACUC protocols. You’ve Got Questions, We’ve Got Answers!In this issue, we answer questions about submitting protocol violations, reporting animal welfare concerns, and what to do if you suspect Research Misconduct. Export Controls and Sponsored Projects: Why Even ‘Basic Research’ Needs Compliance Review!In this special article, we provide Guidance and Best Practices for Ensuring Your Submission Compiles with Research Compliance Regulations and Processes. ORC CalendarFind all of the meeting and deadline dates for the next three months of Board and Committee meetings.
|
|
What’s Happening in the ORC?Here is the latest news from the ORC: On April 17th, the ORC will be featured at the HSC Research Café, hosted by Dr. Paula Gregory! Our very own Ms. Eleanor Knutson will be the guest speaker, and will be presenting, Getting to the Yes: How to Navigate the Research Compliance Regulatory Landscape. Please join us virtually via Zoom from 1:00 to 2:30pm for an introduction to our office and the opportunity to ask us your burning regulatory questions! The IACUC is in the process of transitioning away from the GRAMS/Huron system due to unresolved complications. During this transition period, the IACUC Office will ensure that all protocols previously entered in the GRAMS/Huron system are accessible to researchers. We will provide regular updates on the progress of this initiative. Please feel free to contact the IACUC Office with any questions. Biomedical Research Awareness Day (BRAD) is also right around the corner – April 18th! BRAD is an Americans for Medical Progress program focused on enhancing public understanding of the indispensable role animals play in biomedical research and the consequential strides made in medical science benefiting humans and animals alike. Join us for the 2024 BRAD Webinar: The Importance of Animals in Addressing the Drug Abuse Crisis. It’s Semi-Annual Inspections time again for our animal researchers! Laboratory inspections are scheduled for April 24th. The IACUC office will be reaching out to our researchers with more details as the time for inspections nears. The North Texas Regional IRB is continuing on working towards accreditation efforts! After submitting the Step One application to the Association for the Accreditation of Human Research Protection Programs, Inc. (AAHRPP) in December, the NTR IRB is currently busy responding to AAHRPP’s feedback and preparing for the second phase of the application. We will be sending more updates as we move closer and closer to achieving accreditation, so stay tuned! The North Texas Regional IRB is also very excited to announce that they will have a booth at the Annual JPS Research & Quality Symposium on May 21st. If you’ll be in attendance, come say hi and learn about the many benefits our regional IRB has to offer! Employee Spotlight – Meet Crystal Perez
|
| What do I need to do to add funding to my IACUC Protocol?
Since all IACUC protocols require some type of funding, there are various sources the IACUC will accept as funding for the protocol. The type of funding will dictate what will be required to include in your protocol.
As you can see, adding funding to your IACUC protocol does not have to be a daunting process. Hopefully, this article has provided you with the necessary guidance and answered any questions you may have had. For additional information, a friendly reminder that the IACUC Office is available to answer any questions when funding is received. Feel free to reach out to the IACUC Office the moment you receive the priority score, and we’ll be happy to guide you through the process! |
||
You’ve Got Questions, We’ve Got Answers!Q: How do I submit a Protocol Violation? A: All reports of protocol deviations/violations involving a human subject research project should be submitted to the North Texas Regional IRB within 10 working days of discovery. Protocol deviations/violations must be submitted to the NTR IRB in the IRBNet system. Instructions for submitting a report of a protocol deviation/violation in IRBNet are provided below.
The NTR IRB’s protocol violation guidance document provides definitions and examples of protocol violations and deviations and also describes how to minimize the likelihood of such events.
For answers to questions about protocol violations/deviations, please contact the NTR IRB staff at NorthTexRegIRB@unthsc.edu.
Q: How do I report an animal welfare concern? A: An Animal welfare concern can be reported to any IACUC member or directly to the IACUC office via e-mail at IACUC@unthsc.edu. Anyone with an animal welfare or non-compliance concern can exercise their right to anonymity. Anonymous reports can be submitted through the HSC Trust Line (844-692-6025), or to the IACUC Office, with a note that you wish to remain anonymous. Anyone who reports a concern is subject to whistle-blower protections with no threat of reprisal. Reportable animal welfare concerns include (but are not limited to):
The Office of Laboratory Animal Welfare list of reportable situations is a suggested reference point for animal welfare concerns that must be reported to the IACUC.
Q: What Should I do if I suspect Research Misconduct? A: Federal regulations (set forth at Title 42, Part 93, Subpart A, 93.103) define Research Misconduct as “fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.” While the federal regulations mainly apply this to research that is funded by the Public Health Service (PHS), at HSC, our Research Integrity/Misconduct policy and processes apply to ALL research (regardless of funding).
If you suspect or have witnessed that fabrication, falsification, or plagiarism has occurred in the conduct of research being done at HSC, we encourage you to report this! Reports of potential research misconduct can be made to the HSC Vice President for Research, to the Provost, or to the Research Integrity Officer (within the Office of Research Compliance). It is important to note that due to the sensitive nature of research misconduct issues, and given they can have a severe impact on the reputation and career of the person who the report is being made about (aka, “The Respondent”), it is important that the individual making the report (aka, “The Complainant”) does so in good faith.
Once an allegation is received, it will be assessed to determine whether it falls within the policy and regulations and warrants further formal review – this is referred to as an “Inquiry”. If determined that an Inquiry needs to be completed, the purpose of the Inquiry is then to determine if a formal “Investigation” into the matter needs to be completed.
You can review our HSC Policy 8.106 Research Integrity as well as the HSC Research Integrity Procedural Manual for further information and guidance. Please also review the Research Integrity and Misconduct webpage, within the HSC Office of Research Compliance site for additional information and training.
The U.S. Department of Health and Human Services Office of Research Integrity (DHHS ORI) website is also a fantastic source for guidance on handling research misconduct issues and includes tools/resources for institutions.
*For overall guidance and training on Responsible Conduct of Research, which includes Research Integrity, we encourage you to complete the HSC CITI Course, “Responsible Conduct of Research”.
As always, any specific or general questions regarding research misconduct can be sent to the Office of Research Compliance at Research.Compliance@unthsc.edu or call us at 817-735-0409. Any other general questions or not sure where to start? You can visit our Office of Research Compliance webpage, call us at 817-735-0409, or email us at Research.Compliance@unthsc.edu. We look forward to hearing from you!! 😊 Have a question you would like answered? Just click the link below to submit your question. |
||
Export Controls and Sponsored Research Projects: Why Even ‘Basic Research’ Needs Compliance ReviewExport Controls and Sponsored Research Projects: What Researchers Need to Know Researchers at HSC conduct a wide range of research projects, but even those classified as fundamental research need to consider export control regulations. Export control regulations, like the Export Administration Regulations (EAR), administered by the Bureau of Industry and Security (BIS), and the International Traffic in Arms Regulations (ITAR), administered by the Directorate of Defense Trade Controls (DDTC), require reviews of university-sponsored research projects, including those classified as fundamental research. This often comes as a surprise to investigators.
These regulations protect national security and economic interests and apply to the transfer of sensitive technologies, information, and equipment to foreign countries or individuals. By understanding export controls, we ensure HSC’s innovations stay focused on improving lives.
Key Areas Where Export Controls Might Affect Your Project: Export controls are complex regulations that directly impact sponsored research projects at HSC. While not every project requires a comprehensive review, the Office of Research Compliance (ORC) is available to assist researchers, departments, and principal investigators (PIs) with export control concerns. When a project is referred to the ORC for review, the office assesses several key areas to ensure compliance with export control regulations:
Researchers, departments, and PIs are encouraged to consult with the ORC if they have any questions or concerns about potential export control issues in their sponsored research projects. By proactively seeking guidance from the ORC, researchers can ensure their projects remain compliant with export control regulations and avoid any potential delays or legal consequences.
Researcher Responsibilities: Early Disclosure: Include details such as the SOW (scope of work), international collaborations or travel in your initial project proposal. Understand Restrictions: Familiarize yourself with the export control regulations specific to your funding source and any relevant OFAC sanctions. Timely Submission: Allow sufficient time for ORC review to meet project and funding deadlines. Maintain Records: Keep thorough records demonstrating your sponsored project’s compliance with export controls.
Additional Resources:
The ORC is Here to Help: The Office of Research Compliance (ORC) offers guidance throughout the export control process to ensure your sponsored research projects remain secure and compliant. If you have any questions or concerns about potential export control issues in your sponsored research projects, please contact the ORC for help.
Contact: For questions or assistance, please reach out to Crystal M. Perez, D. Bioethics, MS, CHW crystal.perez@unthsc.edu or the ORC office at research.compliance@unthsc.edu |
||
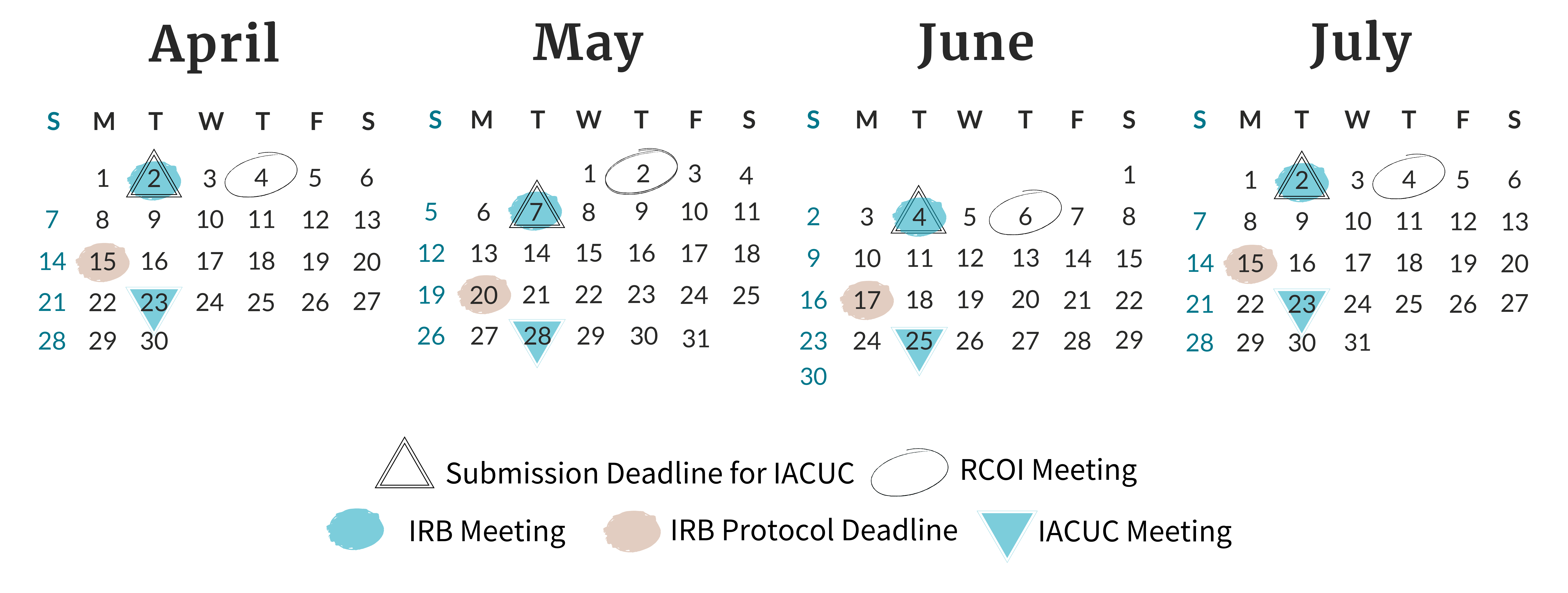
ORC Calendar |


Social media