Research Interests
Transcriptional and post-transcriptional regulation of MIEN1 in Prostate and Breast Cancer
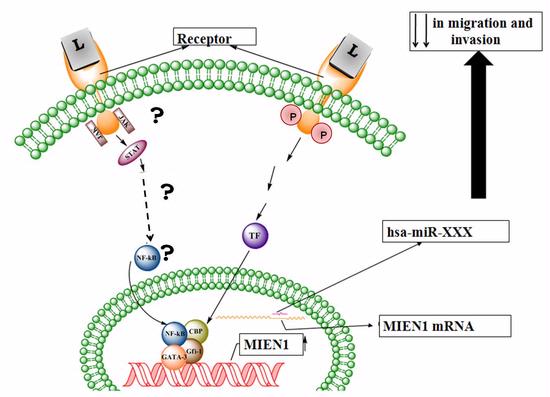
MIEN1 is a novel gene located in the 17q12 region of the human chromosome. It is over-expressed in cancer and there is minimal to no expression in normal cells and tissues. It has also been shown that MIEN1 is mostly co-amplified with Her-2/neu, but there is also independent expression of MIEN1. In the past, our lab has shown the role of MIEN1 in metastasis of prostate cancer. This project revolves around identifying the commonly studied gene regulatory mechanisms, microRNA and transcription factors, in the regulation of MIEN1.
Role Of miRNA in regulation of annexin A2 level in early and metastatic prostate cancer
My project is based on the possible role of micro RNAs in the regulation of Annexin A2 levels in hormone dependent and hormone independent stages of prostate cancer. Initially I plan to look at the possible role of the micro RNAs in various prostate cancer models and once established, I plan to look at whether these micro RNAs can be used as a diagnostic tool in prostate cancer patients. Within this domain, I also plan to look at the possible transport of these regulatory micro RNAs from one cell to another, thereby regulating the levels of Annexin A2 and other key proteins, which in turn can regulate angiogenesis and metastasis.
Nanoparticulate drug delivery system for active targeting of therapeutic agents in cancer therapy
Chemotherapeutic agents have been most promising therapy for treatment of late stage cancer patients. However, a major setback for efficiency of these agents is the adverse side effects and/or resistance. One can dramatically increase efficiency of these agents by reduction of its exposure to non-specific sites. My research focus is to develop novel drug delivery systems (NDDS), which can be tailored to preferentially deliver these
agents, individually or in combination, to tumor specific sites. We have developed and validated a novel way of surface functionalization of PLGA nanoparticles by non-covalent insertion of a chemical cross-linker, BS-3. We have validated this technique by using an antibody as a prototype ligand. In my present study, I am exploring the use of this drug delivery system to preferentially deliver a combination of chemotherapeutic agents to bone marrow microenvironment. We have been successful in formulating bisphosphonate coated nanoparticles which can preferentially localize to bone marrow. We are using different in vitro and in vivo systems to validate our hypothesis and efficiency of such drug delivery systems in treatment of cancer patients. Development of such a drug delivery system to target bone metastasizing cancers, such as breast, prostate, and multiple myeloma, can be used to decrease the proliferation of cancer cells in bone and resulting complications. Co-encapsulation of different drugs, which act synergistically, in such bone targeted nanoparticles will further enhance its therapeutic efficiency as well as decrease the required dose and dose frequency due to its sustained release property.
Novel Gene MIEN1 as Therapeutic Target in Breast Cancer
A novel gene MIEN1 (C17orf37) has emerged as a potential diagnostic biomarker of metastatic breast cancer. Located in the chromosomal region 17q12-21 along with MIEN1 are several important genes, GRB7, STARD3 and HER-2/neu oncogene which are frequently amplified in breast cancer. In normal breast tissues MIEN1 is either completely absent or low, with a robust expression in invasive breast tumors. Recently shown by our Laboratory is the role of MIEN1 in promoting migration and invasion of cancer cells.MIEN1 shows significant promise as a novel gene and understanding of the role of this protein in breast cancer will validate its use as therapeutic target. The second most common cause of cancer death in the U.S., breast cancer’s mortality rates remain high due to its metastasis. Recognition of cancer-specific biomarkers has tremendous potential to enhance detection, treatment, and prognosis of the disease.
Optimization and scale-up of curcumin loaded nanoparticle formulation
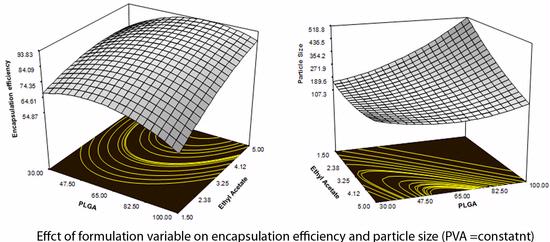
The objective of this study was to optimize and characterize the formulation of curcumin loaded poly (lactic acid-co-glycolic acid) (PLGA) nanoparticles, to determine the effect of formulation variables such as amount of PLGA, concentration of stabilizer and the volume of organic phase and their influence on the physiochemical properties of the nanoparticles. The responses evaluated were particle size, polydispersity, encapsulation efficiency and percentage drug loading. We applied the Reponse Surface Methodology (RSM) using Central Composite Design (CCD) model to optimize the formulation of PLGA-CURC. An analysis of variance was performed to determine response surfaces. Furthermore, the desirability function approach was applied to obtain the best optimized solution among the multiple responses.
Development of Annexin A2-targeted curcumin loaded multifunctional PLGA nanoparticles for breast cancer therapy
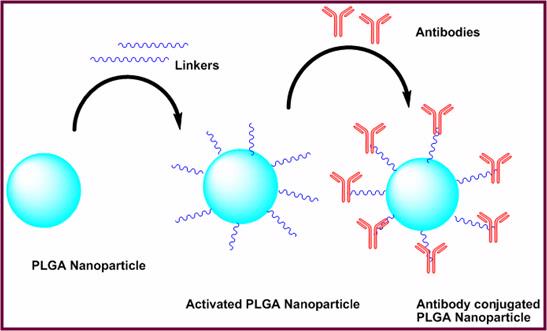
The proposed project uses an innovative approach by formulating and validating the efficacy of a novel drug delivery system that utilizes a multi-target anticancer drug, curcumin, encapsulated within PLGA nanoparticles conjugated to annexin A2 antibody for the delivery of curcumin to breast cancer cells. Considering the recent findings that annexin A2 as a modulator of breast cancer metastasis, we are proposing combination treatment strategy using curcumin loaded annexin A2 targeted PLGA nanoparticles. The monoclonal antibody against annexin A2, besides targeting the nanoparticles at the desired site, would also inhibit annexin A2 dependent plasmin generation, which affects the invasion and migration, required for neoangiogenesis in highly invasive and metastatic breast cancer cells. This combination strategy aims to target cancerous cells most capable of metastasis and functionally inactivate the cell using curcumin in a localized fashion, thus reducing overall efficacious dose necessary as compared to free curcumin. We hypothesize that poly lactic-co-glycolic acid (PLGA) based nanoparticles encapsulating curcumin that are selectively targeted to annexin A2 cell surface antigen will synergistically inhibit tumor growth and progression and serve as more efficacious therapeutic agents in the treatment of metastatic breast cancer. This form of combination treatment strategy as an adjuvant to the traditional chemotherapeutics or surgical resection would advance treatment options for oncologists.

Social media