Kejin Hu Lab
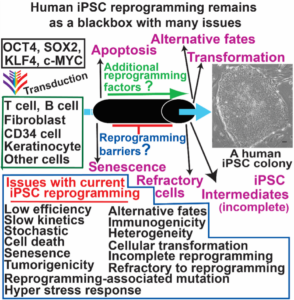
Illuminating the human iPSC reprogramming black boxOverexpression of OCT4, SOX2, KLF4 and MYC can convert many types of human cells into embryonic stem cell like-cells, called induced pluripotent stem cells (iPSCs). Human iPSCs have been an excellent tool for basic research, drug screening, disease modeling, biotechnologies, and cell therapy. The current iPSC reprogramming remains as a black box to scientists as it is still inefficient, slow, and stochastic with additional issues of apoptosis, senescence, mutagenesis, reprogramming stress, incomplete reprogramming, and unwanted cell fates (See Figure). My lab has been tackling these issues associated with the current reprogramming technologies. To this end, we use approaches of library screening, lentiviral vector, shRNA knockdown, CRISPR knockout, RNA-seq, ChIP-seq, ATAC-seq, mutagenesis, bioinformatics, and others. |
 |
Roles of BET proteins in human iPSC reprogramming and inflammation
Bromodomain extra terminal (BET) proteins bind to acetylated chromatin, and thus regulate transcription. My lab identified BRD3R as a new reprogramming factor after extensive library screening. We also discovered that mild chemical inhibition of BET proteins promotes iPSC reprogramming. Our subsequent research unexpectedly revealed that dominant negative mini BET proteins lacking the bromodomain and ET tail enhance iPSC reprogramming mainly by mitigating reprogramming stress. However, the underlying molecular determinants for their reprograming activities are not known. My lab is studying the hidden reprogramming activity in BET proteins (BRD2, BRD3 and BRD4). We also study BET regulation of inflammation using iPSC-based cellular model and conditional knockout mouse models.
Alternative inhibition of BET functions
BET inhibition is a promising pharmaceutical approach to treat cancers and other diseases. However, current chemical BET inhibitors inhibit all BET members indiscriminately by targeting their bromodomains. Current chemical BET inhibitors are toxic, for examples, BET inhibition causes thrombocytopenia, neutropenia, anemia, testicular and gastrointestinal toxicities. Over the years of dissecting roles of BET proteins in reprogramming, we found that mini BET proteins of BRD2, BRD3 and BRD4 act dominant negatively. These mini BET proteins lack the characteristic bromodomain and the ET tail. Our data indicate a new mechanism of BET inhibition since these BET fragments contain no bromodomain. We are investigating how these mini BET proteins inhibit BET functions.


Social media