|
Find the latest updates on all areas of the ORC, including the IACUC, the NTR IRB, RCOI, and International Compliance.
This quarter we introduce our ORC member – Alyson Stearns!
In this issue, we’ll help you get to the “Yes” with your time-sensitive human subjects, animals subjects, or export-controlled activities.
In this issue, we answer questions about who can be the Principal Investigator or listed as Key Personnel on IRB and IACUC protocols.
In this special article, we’ll discuss tips and strategies for effective recruitment in your Human Subjects Research.
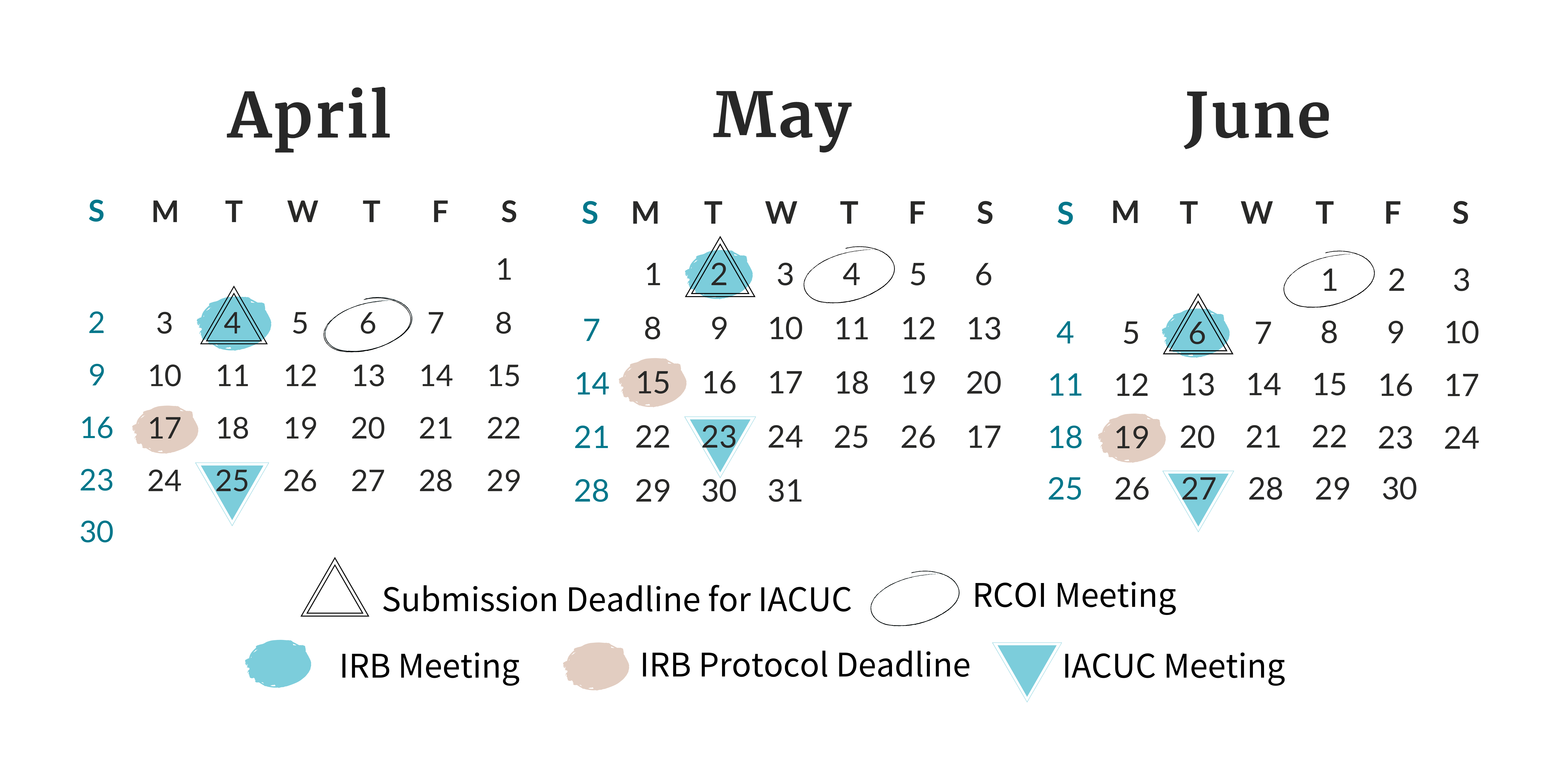
Find all of the meeting and deadline dates for the next three months of Board and Committee meetings.
|
|
What’s Happening in the ORC?
Here is the latest news from the ORC:
- Karina Garcia, our IACUC Administrator, left the institution at the end of December. Our office is currently looking to hire a new IACUC Administrator.
- Despite current staffing shortages, the IACUC Office was busy with preparations for the AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) triennial re-accreditation site visit, which took place on March 17th. The hard work and efforts of the IACUC and DLAM offices paid off though, and AAALAC recommended Continued Full Accreditation of our HSC Animal Care and Use Program (note that the formal re-accreditation will be received after the AAALAC Council meeting in May).
- Additionally, the article that our IACUC office was involved in for Lab Animal Magazine, (titled Once Used, Twice Counted), has been published and can be accessed here.
- Our office has continued to work diligently on updating current/creating new procedures, webpages, and guidance materials in order to better serve the research community:
-
- We now have an Off-Boarding Information for PIs/Researchers webpage to provide guidance to Principal Investigators who are leaving HSC. Specific details for each Research Compliance area that might affect your research (e.g., IACUC, IRB, Export Controls, etc.) are outlined on this page.
-
- The IACUC has updated their Laboratory Posting documents – there is now a separate poster for Emergencies (“Report an Emergency”) and for Concerns (“Report a Concern”). These PDF posters can easily be downloaded and printed so you can keep copies in your lab!
-
- The NTR IRB has created a webpage for Student Researchers, titled IRB Guidance for Studentswhere students will find answers to our most commonly asked questions.
- We are continuing to work with other colleagues across the University on improving practices and processes for our researchers and making additional guidance and resources available…so stay tuned!
back to top
Employee Spotlight – Meet Alyson Stearns 
How long have you worked in the HSC Office of Research Compliance? What is your role, and what do you like best about it? Well, this time around I have worked as an IRB Compliance Manager with the ORC since February of 2022, but because I worked here previously, you could say I am a repeat customer, ha. I first began my career at UNTHSC as a Research Compliance Manager in August 2019. Several months later, this tiny thing called the COVID-19 pandemic happened, which temporarily derailed all my carefully made plans; instead of reviewing research projects, I was suddenly attempting to teach my daughter second grade. However, I knew that I wanted to return to the ORC if I had the chance once the world stabilized. I was able to do just that in early 2022, and I haven’t looked back since.
This leads me to what I like best about my role here: my awesome team & our researchers. It is a wonderful feeling to come to work every day knowing that I’m part of a team that has integrity, is kind and supportive, and who really cares about our researchers and the work that we do. Those reasons are why I knew I wanted to come back to the ORC after my “COVID sabbatical.”
Of the following values, which one do you believe you exemplify the most, and why?(Courageous Integrity, Be Curious, We Care, Better Together, and Show Your Fire!) It’s kind of funny, actually… Several of my colleagues mentioned that they thought I should choose better together as soon as I was selected for this newsletter’s Employee Spotlight. They’re absolutely right, though; I truly believe that we are all better together, and that diversity of thought, experiences, cultures, and lifestyles enriches everything, especially in the workplace. Every person that we work with has a unique perspective from which we can learn. I view it as my personal mission to encourage my colleagues, to collaboratively find solutions to problems, and to be an ally to our researchers in their quest for compliant, ethical research with human subjects.
What is something we would be surprised to find out about you? My team already knows this, but I took classes at the Dallas Comedy House before it closed and performed improv on stage in front of a live audience. I also love plants and have given cuttings of the pothos in my office to fellow colleagues. And finally, I originally had six wisdom teeth instead of four, leading me to believe that my superhero name would be Jaws.
back to top
|
|
|
How to Get to the Yes
Time-sensitive human subjects, animals subjects, or export-controlled activities
Human Subject Research Projects:
If you have a time-sensitive submission (e.g. a new research project that has specific start date; a modification to an existing, IRB-approved project involving activities that need to occur by a certain date, etc.), there are some important steps you can take to help ensure your submission is reviewed and approved by the NTR IRB within the necessary timeframe.
- Plan Ahead! Know any deadlines that are associated with your project (whether they be from the funding agency/sponsor, from your department, etc.) in advance so you can plan your submission accordingly. If the project presents greater than minimal risk to research participants thus requiring Full Board review (review by the convened Board at an IRB meeting), it is important to know the IRB’s deadlines for submitting materials for review at a convened meeting. The NTR IRB’s review schedule, including the corresponding submission deadlines for each IRB meeting, is available on the NTR IRB’s Review Schedule page.
- Inform the NTR IRB about the time-sensitive nature of the submission, including any deadlines that you hope to meet, and include an explanation of the time constraint(s). If you are submitting a new research project for review, you will be asked (in the IRBNet Wizard application form) to provide information about the deadline. For other types of time-sensitive submissions (e.g., requests for protocol modifications), please include a message in the IRBNet system when you submit the package for review, notifying the IRB of the time-sensitive nature of the submission.
- Prior to submitting a new project to the NTR IRB, ensure that all key personnel have completed adequate training in human subject research (such as the CITI Basic Course in Human Subject Research).
- Submit the materials to the NTR IRB with sufficient time for review. Note that the amount of time needed for review depends upon several factors, including the level of IRB review required for the submission; the complexity, quality, and completeness of the submission; and the current workload of IRB staff.
- Submit a complete package in IRBNet that includes all the required documents.
While the NTR IRB cannot guarantee that a time-sensitive submission will receive IRB approval by a certain date, the NTR IRB strives to accommodate investigators’ deadlines whenever possible and reasonable.
Animal Research Projects:
Oftentimes, sponsor-initiated projects cause a time-sensitive concern for IACUC submissions, such as a new protocol, or an amendment to an approved IACUC protocol. Here are some helpful tips to help navigate receiving IACUC approval within the necessary timeframe.
- When? When planning time-sensitive research projects, it is good to know the IACUC Submission Deadlines and Meeting Dates. Knowing this can help you achieve submitting your project or project modification in a timely fashion for full committee review.
A note in regard to amendments: Not all amendments need to go through full committee review, which may help in a quicker turnaround time for the review process.
- Where? All protocols and amendments are submitted through the GRAMS system. The Forms Page on our website provides a link to the GRAMS system, quick reference guides, and protocol writing assistance tools.
- What? It is important to include all appropriate information in your submission, to help facilitate a smooth IACUC review. If you are unsure what is needed, feel free to visit the IACUC SOPpage, which provides information designed to help you with writing your protocol, while ensuring you are staying within the federal regulations as well as the guidelines of the HSC Animal Care and Use Program.
- Who? It is always helpful to inform the IACUC Office when submitting time-sensitive projects, including any deadlines needing to be met. If major changes are being made to a project, or you are uncertain how to go about submitting your new protocol, feel free to set up a consultation with the IACUC Office. This may also help in ensuring the application is complete prior to submitting. This can be done by completing the IACUC Consult Request Form.
While the IACUC Office cannot always guarantee that a time-sensitive submission will receive IACUC approval by a certain date, we will do our best to work with our researchers in hopes of meeting the identified time constraints of the project.
Compliance Review for Export Control Regulations on International Travel:
As we approach a new season of international travel, we would like to remind you of the requirements for all University-related travel outside of the United States. Here are some guidelines to keep in mind:
- Plan ahead and submit your International Travel Request in Concur at least 90 days in advance to allow sufficient time for the Export-Control compliance review process. This review is required for all international travel.
- Complete and submit an International Device Checklist to the Export Controls Officer for all international travel requests. This is required to ensure that all electronic devices, equipment, and technology being taken outside of the United States are in compliance with export control regulations.
- If your international travel involves time-sensitive activities, such as conferences, meetings, or research collaborations, inform the Export Controls Officer as soon as possible. This will allow the Export Controls/International Compliance Office to take appropriate measures and ensure that your travel plans are in compliance with export control regulations.
- Provide the Export Controls Officer with a detailed itinerary of your travel plans, including any planned activities that may require the temporary export of controlled items. This will help the Export Controls/International Compliance Office to determine the appropriate level of review needed for your travel.
- For more information on HSC international travel compliance, please visit our webpage. This resource provides additional information on international travel compliance and requirements.
By following these guidelines, you can help ensure that your international travel for University-related purposes is in compliance with export control regulations, while also avoiding any potential delays or complications. Please do not hesitate to contact the Export Controls/International Compliance Office if you have any questions or concerns regarding the process.
back to top
|
|
|
You’ve Got Questions, We’ve Got Answers!
Q: Who can serve as PI? (and who cannot serve as PI)
A: For the IRB: The term “PI” (Principal Investigator) implies specific responsibilities and interactions with a research project. The PI is responsible for all overall aspects of the research project (which include the scientific, technical, and administrative aspects), even when certain responsibilities have been delegated to their staff, students, or co-investigators. To be a Principal Investigator of a human subject research project, the individual must be:
- Full-time and/or part-time regular and/or non-regular faculty of HSC.
- Full-time or part-time non-faculty employee of HSC who is NOT a student at UNTHSC. Note that students and Adjunct Faculty cannot serve in the capacity or role of Principal Investigator.
- Faculty or staff associated with an affiliated institution of the NTR IRB (JPS, UNT Dallas, Cooks Children, Tarrant County Public Health).
For the IACUC: For protocols using animal subjects, HSC Faculty are eligible to serve as a Principal Investigator for an IACUC Protocol. However, individuals without HSC Faculty status may serve as a PI under the following conditions: (1) as a co-investigator with an individual who meets the eligibility requirements, or (2) receive written approval from the Executive Vice President for Research.
Q: Who can serve as key personnel? (and who cannot serve as key personnel – minors)
A: For the IRB: All research personnel who are actively involved with the project and anyone who will be interacting with human subjects or their data from a research perspective are considered “key personnel”. Key personnel do not have to be affiliated with the NTR IRB, but they are required to complete the necessary training (e.g., CITI Human Subjects Training, Good Clinical Practice) and to submit Conflict of Interest Statements if involved in an Expedited or Full Board protocol.
Minors cannot be named as key personnel in protocols. If undergraduate students (who are 18 years of age and over) will be involved in research protocols, please reach out to the NTR IRB for additional requirements.
For the IACUC: Anyone who will be working on the design, conduct, or reporting of the research for a specific project/activity must be listed in the “Protocol Team Members” section of the protocol (in the GRAMS system). This applies to individuals who are internal or external to the institution and includes faculty, staff, and students. In addition to the PI, all individuals listed on the protocol must first complete the Personnel Requirements, which include the completion of appropriate training and enrollment in our Occupational Health Program.
back to top
|
|
|
Recruitment Tips for Human Subjects Research
Researchers might wonder or ask themselves…Why is the IRB so interested in my recruitment materials? One may think study recruitment involves harmless informal interactions with potential research participants – however, recruitment is an important aspect of human subject research because it is considered part of the informed consent process. The information participants are given during the recruitment phase can make a huge impact on their decision to participate; thus, study recruitment should be approached with care. All recruitment efforts must respect personal rights to privacy and confidentiality, be compliant with the relevant regulations (Federal, HIPAA, FERPA) as well as institutional policies, and avoid possible coercion or undue influence (e.g., power differential between the researcher and the subject, therapeutic misconception, overly enticing incentives and false advertisements). For this reason, the IRB assesses study recruitment materials to ensure that the rights and welfare of potential participants are protected from the very first contact with the research team.
Of course, study recruitment strategies must be effective in attracting potential participants in addition to being accurate and ethical. When developing recruitment materials and strategies, make sure to give special considerations to the following areas to ensure potential research participants are appropriately receiving and understanding your message:
- Target population: There are several factors that influence how a message is received and understood by potential subjects: age/generation, culture, race/nationality, gender, language, and current / previous geographical location, to name a few. Researchers may consult with community stakeholders (e.g., pastors, community leaders/elders, teachers, local ethnic centers and resources, etc.) or create an advisory board to assist in their understanding of the subject population.
- Content: Provide a concise, meaningful explanation about the research study and any relevant inclusion criteria along with instructions for participation and contact information for the research team. Note it should be clear to individuals that they are being approached for research purposes.
- Format: Present the information in a clear, uncluttered, and easy to understand manner; add items of interest to capture the attention of your audience, such as (appropriate) graphics and videos.
- Delivery method: Consider the format of your research activity along with the best way to reach your target population. Passive forms of recruitment, such as online/social media or email, can be effective for online surveys; paper flyers or mailed postcards may be better suited to reach individuals lacking access to computers or an internet connection. Alternatively, active forms of recruitment, such as (physician or community) referrals or in-person delivery, are more effective for research studies with a unique subject population or which require a high level of subject engagement.
- Location: Where appropriate and permissible, place research advertisements in a visible, high-traffic location. For in-person recruitment especially, be respectful of the potential subjects’ privacy and current personal state (emotional, mental, and physical).
Individuals are more willing to participate in research when researchers carefully tailor the recruitment message to meet their unique needs and situations. In respecting participants and their circumstances, researchers build rapport with the intended subject pool and create strong relationships that translate into better research outcomes.
Because the IRB must review and approve all recruitment methods and materials for regulatory compliance and ethical considerations, it is important to submit appropriate documentation for each recruitment method to the IRB, including any scripts (oral, telephone, video, radio, etc.), flyers, advertisements, guides, handouts, and any other relevant materials. For a more thorough explanation about what must be submitted to the IRB for study recruitment, please visit the NTR IRB’s Recruitment Guidance document that is accessible from the Guidance and Sources page on our website.
Please note that the Principal Investigator is responsible for ensuring that study recruitment materials follow all institutional guidelines. As always, please contact the NTR IRB with any questions or suggestions at NorthTexRegIRB@unthsc.edu.
back to top
|



Social media