Focusing on Research – September 2018
September 18, 2018 • Focusing on Research
Research Development & Commercialization
Currently, there’s a buzz around the campus for the ~$2.5M investment in fostering research core infrastructure. It is happening! With your help, we will continue to be strategic, justify ongoing investments and most importantly, continue delivering results. We are committed to invest in research infrastructure! President Williams also reiterated this commitment at the Town Hall.
- A new high-resolution confocal microscope will soon be purchased for the Microscopy Core Facility. To aid in that purchase decision, three candidate instruments were invited for demonstrations on campus. Zeiss was on campus in August to demo the LSM 880 Confocal Microscope with Super-Resolution Airyscan Module. This month, Nikon will demo its A1R-MP+ Multiphoton System, and Leica will demo its DMI8 SP8 DIVE System in October. Hands-on demonstrations using investigator or vender provided samples are available for small groups in 90-minute intervals. For more information, please watch the Daily News and Microscopy Core Facility website.
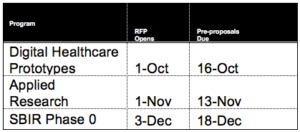
- Cycle 2 of the Research and Innovation seed grant programs has launched. The Basic Research RFP opened September 4; pre-proposals are due September 18. Other competitions this fall will include those listed below. For more information, see https://unthsc.infoready4.com.
- UNTHSC is hosting Ignite DFW 10 at the MET on October 17th from 6:00-8:00pm. Ignite is comprised of short, TED style talks, so each speaker only gets 5 minutes. Speakers get 20 slides that automatically advance every 15 seconds. The goal is to give the audience a thought-provoking idea or topic and then build a presentation around that thesis. The tone “can vary from serious to funny to interactive – totally up to the speaker.” This will be the first time this event has been held in Fort Worth and is one more way we are trying to engage with larger entrepreneurial community.
- Contact Cameron.Cushman@unthsc.edu for a discount code for free tickets for faculty and students.
- Elsevier Pure Portal Research Expertise Database. UNTHSC is establishing Elsevier Pure as a tool to improve the face of faculty on campus and showcase their expertise alongside our Profile system. Currently, approximately 310 profiles for UNTHSC faculty are populated from Scopus, Profile and HR system resources. Stay tuned, we’ll roll it out for faculty review by September end.
- Newly Available Campus Resources to support your research through the UNT System College of Pharmacy, The Discovery Cores and Exact Diagnostics.
- Training Opportunities are announced in the Daily News. There are several research training opportunities available for students and early career investigators in all our schools. These include, Intramural NIAID Research Opportunities (INRO), American Association for the Advancement of Science, Katie Award in Environmental Health Practice Research, and the Grants-in-Aid of Research (GIAR) program.
That’s about Research Development initiatives. Here’s how we are trying to facilitate Research Administration to reduce the administrative burden on PIs and invest in continuous improvements.
Research Administration – Office of Research Compliance
- We got IRBNet: The North Texas Regional IRB recently invested in a new electronic IRB protocol submission and review system – “IRBNet” to streamline human subject research workflow process and make the investigator-IRB experience smoother and faster. Be sure to register yourself on the IRBNet.org website.
- Effective September 1, 2018 all NEW protocols submitted for IRB review will need to go through IRBNet. Continuing reviews and amendments will be accepted via IRBNet after October 1.
- Questions about IRBNet or IRB in general? Contact the North Texas Regional IRB Office at (817) 735-0409, or email NorthTexRegIRB@unthsc.edu
- IRB Reciprocity Agreements: To help minimize the burden of multiple IRB approvals from several entities for a single multi-site project, the North Texas Regional IRB developed reciprocity agreements with several institutions and systems. In addition to a major IRB relationship with John Peter Smith Health Network (JPS), we recently entered into an agreement with Medical City North Texas to allow for single IRB review at both sites. Currently, we have reciprocity agreements in place with the following institutions:
- Baylor Research Institute; Centers for Disease Control & Prevention; Cook Children’s Health Care System; Copernicus Group; North Texas Medical City (Formerly Medical City Dallas Hosp); Quorum Review Inc.; Schulman Associates Institutional Review Board; UT Arlington; UT Southwestern Medical Center at Dallas; Univ. of Washington and Univ. of Wisconsin-Madison (Health Science)
- Contact the North Texas Regional IRB Office at (817) 735-0409, or email NorthTexRegIRB@unthsc.edu for additional details.
- Research Conflict of Interest (RCOI) Update: It is that time of the year, again! September is “research conflict of interest disclosure” month. NEW this year – the RCOI annual training combined with the actual disclosure(s). Simultaneous training and disclosure, is a more effective way of meeting federal agency and institutional research compliance commitments. Use the RCOI weblink and complete this annual process.
- UNTHSC joins SMART IRB Recently, UNTHSC completed all steps to join the NIH-initiated “SMART IRB” Reliance program. This SMART IRB program is designed to meet the NIH-mandated “single IRB” review system for NIH-sponsored research projects. As a member of this reliance agreement, UNTHSC investigators may rely on the IRB review and oversight from another SMART IRB member institution for NIH-funded projects. For more details on the SMART IRB program and procedures and process, contact Dr. Brian Gladue, Associate VP for Research Administration at (817) 735-5083.
Research Administration – Office of Sponsored Programs (OSP)
As we embrace a culture of compliance and proactive research risk management, we strive to diminish the administrative burden on our researchers. The OSP sends out newsletters to the UNTHSC Research Community informing them of our continuous improvement initiatives.
- Monthly OSP Report Now in PowerBI: We have been listening to your feedback and have added some new functionality into the Monthly Report. Now you can see a 3-year trend of UNTHSC’s awards and proposals data. We also added a new tab which contains all monthly report table data to make it easier to gather comprehensive data without having to pull separate reports from each tab. If you would like to dig into your data in Power BI, but don’t know where to start, we have a handy instruction guide here.
- Revised F&A Voluntary Waiver Request Process: Facilities and Administrative (F&A) costs are real expenses. From the salary of the accountant who draws down funds and the admin who submits ePars for your project, to the electric bill to keep lights on in the buildings, F&A covers a wide range of expenses that are difficult to allocate accurately to individual projects. The Office of the VPR receives requests to voluntarily reduce or waive our F&A rate for cause. This process has been updated to allow for thoughtful consideration of each request based on the merits and will facilitate data driven decisions and constructive dialogue. Please follow the revised F&A Voluntary Waiver Form:
- Submit at least two weeks before the proposal deadline
- Include two (2) copies of the detailed budget: one (1) at the full allowable F&A rate, and one (1) at the requested reduced rate.
- Include a detailed justification for the request.
- Obtain signatures from the PI, the Department Chair, the Institute/Center Director (if applicable), and the Dean. By signing, each of these individuals agree to waive their F&A return investments to offset the institutional loss of these expenses if the voluntary waiver is approved.
- Questions about the revised process? Feel free to reach out to the Office of Sponsored Programs at 817-735-5073.
Celebrating Research
Research achievements and collaborations information gathered via the last VPR newsletter, Daily News & email requests and Scopus are all compiled. If you have anything research to celebrate, please email ResearchRelations@unthsc.edu. Current sections include UNTHSC Newsroom – Research, Notable Collaborations, Recent Publications and Notable Honors.
President’s Council on Research
This summer, the Research Council was tasked with optimizing processes in how we use one of our key strategic resource, research space. A Spring 2018 research space survey performed with our outside consultant Jim Vitale was fundamental to HSC’s F&A rate negotiations with the federal government and helped link all our research space to funded sponsored projects. The Council recommended that research faculty validate their space data through a survey. The council with review the survey data and work with a collaborative taskforce to develop guidelines and SOPs to implement new space allocation program. More information can be found here.
Meet the Division
In an effort to help you get to know us a little better, we are (re)introducing our staff members in each newsletter. Meet Suzy Griffin and Svetlana Pitts, Program managers for the seed grant program. Dr. Pitts also manages PIVOT, a powerful tool that combines funding and collaboration opportunities for faculty, research staff and students across all disciplines. Pivot is free to all HSC members. Contact Svetlana Pitts at Svetlana.Pitts@unthsc.edu to get started today.
NIH Nexus
- Open Mike: Trends in Diversity within the NIH-funded Workforce. As highlighted in many previous blog posts and the recent National Academies of Sciences, Engineering, and Medicine (NASEM) report, promoting a strong biomedical workforce is a top priority for the NIH. In 2017, NIH launched the Next Generation Researchers Initiative, which is a multi-pronged approach to increase the number of NIH-funded early stage investigators. A key component of this initiative is the call for increased transparency and availability of data about the make-up of the biomedical research workforce. Continue reading
- OHRP Announces Availability of Three Draft Guidance Documents Related to Revised Common Rule. NIH issued guidance for implementing the burden-reducing provisions of the 2018 Common Rule (NOT-OD-18-211). Subsequently on July 20, 2018, the HHS Office for Human Research Protections (OHRP) announced the availability of three draft guidance documents that relate to three burden-reducing provisions Continue reading
- NIH Application Resubmission Policy NIH’s resubmission policy has not changed, but the policy notice (NOT-OD-8-197) highlights some important points:
- Only a single resubmission (A1) of an original application (A0) will be accepted
- An A0 application may be submitted following an unsuccessful A0 or A1 application, with a few exceptions
- What happens when switching FOAs between the A0 and A1 applications? Generally, a change of activity code (e.g., R01) between the A0 and A1 is not allowed, with one exception
- NIH Requests feedback for Prospective Basic Science Studies Involving Human Participants. The “NIH is requesting input on the standards NIH should use in assuring adequate registration and results information reporting for fundamental research studies involving human participants – hereafter referred to as ‘prospective basic science studies involving human participants.’” (NOT-OD-18-217)
- NIH Comment Period: Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules, Streamline Oversight of Human Gene Transfer Protocols. NIH proposes removing the NIH protocol submission, review and reporting requirements under Appendix M of the NIH Guidelines and modify the roles and responsibilities of the Recombinant DNA Advisory Committee (RAC). During this comment period and effective immediately, NIH will no longer accept new human gene transfer protocols or convene the RAC to review individual human gene transfer protocols. The NIH Office of Science Policy will also not accept annual reports, safety reports, amendments etc. for any previously registered human gene transfer protocols. (NOT-OD-18-218)
Once again, don’t forget to take a moment to Give a High5 to our Research Administrators on their special day, Wednesday September 25th!
With Best Regards,
Anuja Ghorpade, PhD
Vice President, Research & Innovation

Social media