Research
SiRNA Delivery for Cancer Therapy
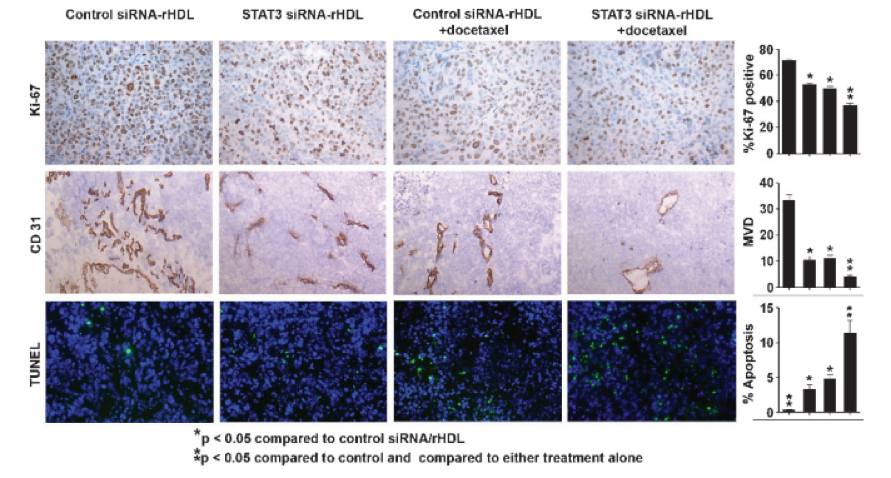
RNA interference holds tremendous potential as a therapeutic approach, especially in the treatment of malignant tumors. However, efficient and biocompatible delivery methods are needed for systemic delivery of small interfering RNA (siRNA). To maintain a high level of growth, tumor cells scavenge high-density lipoprotein (HDL) particles by overexpressing its receptor: scavenger receptor type B1 (SR-B1). In this study, we exploited this cellular characteristic to achieve efficient siRNA delivery and established a novel formulation of siRNA by incorporating it into reconstituted HDL (rHDL) nanoparticles. Here, we demonstrate that rHDL nanoparticles facilitate highly efficient systemic delivery of siRNA in vivo, mediated by the SR-B1. Moreover, in therapeutic proof-of-concept studies, these nanoparticles were effective in silencing the expression of two proteins that are key to cancer growth and metastasis (signal transducer and activator of transcription 3 and focal adhesion kinase) in orthotopic mouse models of ovarian and colorectal cancer. These data indicate that an rHDL nanoparticle is a novel and highly efficient siRNA carrier, and therefore, this novel technology could serve as the foundation for new cancer therapeutic approaches.
Shahzad et al. Neoplasia, 2011, 4, 309-319
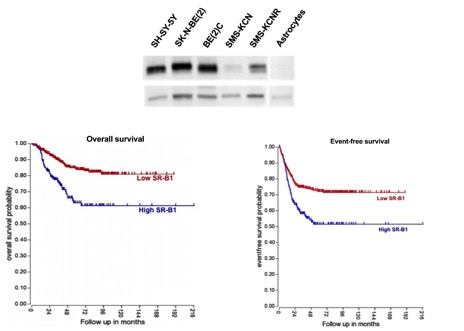
Role of SR-B1 in Pediatric Cancers
Neuroblastoma is an extra cranial embryonal pediatric tumor most prevalent in children less than 1 year. Children diagnosed with high-risk neuroblastoma (HRNB) are unresponsive to aggressive therapy and have about a 50% chance of survival. Similarly, Ewing Sarcoma is equally deadly disease and children diagnosed with metastatic disease have less 30 % chance of survival. Scavenger receptor class B type 1 (SR-B1), a mediator of cellular cholesterol uptake, is overexpressed in and linked to the aggressiveness of many cancers. We are investigating the relationship between SR-B1 and high grade pediatric cancers.
Panchoo et al. BBRC, 2017, in press
Role of SR-B1 and treatment of glioblastoma (GBM) using rHDL nanoparticles
Development and clinical outcomes of many anti-GBM drugs have been thwarted by the inability of these drugs to cross blood brain barrier (BBB) and be bioavailable in therapeutic concentrations in the brain. It is established in the literature that rHDL nanoparticles can cross BBB and can selectively deliver drugs to many brain cancers owing to SR-B1 expression. Endothelial cells lining BBB express SR-B1 receptors as well and can help rHDL nanoparticles to cross the BBB via transcytosis. Currently we are evaluating the potential of these rHDL nanoparticles for GBM treatment.
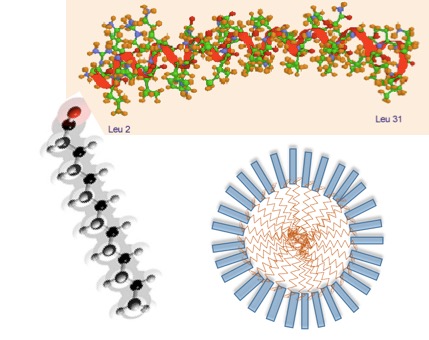
Designing rHDL nanoparticles from Apo –A1 mimetic peptide
Due to the difficulties and associated cost in producing clinical grade ApoA1, We have been exploiting the possibility of using mimetic peptides to synthesize simple nanoparticle formulation of anti-cancer drugs so as to accelerate the development of new drug delivery system to reach clinical stage. Theese peptides are designed based on the known amino acid sequence of ApoA1 protein capable of binding to SR-B1 receptors to facilitate drug uptake. We have several such peptides to design and develop this strategy further enhancing the solubility of many hydrophobic drugs and making them a viable candidate for intra-venous delivery.
rHDL nanoparticles for treatment of solid cancers
Treatment of many solid cancers such as breast and prostate with conventional chemotherapy approaches show severe toxicity issues which results in low quality of life for cancer patients. Reformulating FDA approved drugs with rHDL not only increases the targeted delivery but significantly reduces the off-target toxicity issues. Doxorubicin a known anthracycline widely used in chemotherapy exhibits cardiotoxicity, however data from our laboratory indicates when reformulated as rHDL nanoparticles it significantly reduces the toxicity to normal cells such as cardiomyocytes, kidney cells and astrocytes. Goal of this project is to reformulate various drugs used for the treatment of solid tumors and test its targeting efficiency in-vitro and in-vivo models. Our recent study with triple negative breast cancer has been highlighted as video abstract below.

Social media