Clinical Research Management
UNTHSC MS degree in CRM ranked as #2 best value program in Clinical Research in the nation!
Dorota Stankowska, Ph.D., Director of Specialized Masters Programs
| E-mail: Dorota.Stankowska@unthsc.edu
2023-24 Student Handbook for Clinical Research Management
Program at a Glance
- Semester credits: 49 (on-campus/hybrid cohort) / 37 (online option)
- Average completion time: 18 months with full-time enrollment
- Both on-campus/hybrid (cohort) and online options are available
- Courses are taught by distinguished educators, researchers, and industry experts
- Enrollment for on-campus/hybrid cohort and online cohort at three starting points each year: Fall, Spring, and Summer
What is a graduate degree program in Clinical Research Management?
Clinical research involves the testing and determining the safety and efficacy of new unapproved products, including pharmaceuticals, devices, and biologics in human subjects. Clinical trials in humans (volunteers and patients) are required before marketing approval by regulatory authorities such as the U.S. Food and Drug Administration (FDA). The law that governs clinical research is spelled out in Chapter 21 of the Code of Federal Regulations (CFR). In addition to requiring and legislating clinical trials, regulatory authorities define the standards by which clinical trials are to be conducted. These standards are known as Good Clinical Practices (GCPs).
In-depth knowledge of the CFR and GCP guidelines as well as international guidelines specifically as they relate to the protection of human rights, prevention, and detection of fraud, and the use of sound scientific principles, is a fundamental requirement for clinical research professionals. These individuals are key personnel involved in the conduct of clinical trials which, in turn, are pivotal in getting new products approved and on the market.
The master’s program in Clinical Research Management provides a strong foundation upon which to build or advance a career. The rigorous curriculum focuses on providing students with a broad-based view of the biomedical sciences, as well as in-depth knowledge of regulatory requirements (CFR, GCP), ethical issues, and both the medical writing and administrative skills necessary to conduct clinical research. As part of the program, all students will complete an internship practicum in clinical studies and use this experience to write the internship practicum report pursuant to receiving the Master of Science (MS) degree. The average time to complete the degree is eighteen months with full-time enrollment.
An applicant to the Clinical Research Management program may apply to either the on-campus/hybrid cohort option or the online option. All students complete a six-month internship practicum experience or a capstone project.
Is the program offered on campus or online?
Both the on-campus/hybrid and online options are available to best meet your needs. You must choose to apply to either the on-campus/hybrid cohort or the online track.
On-Campus/Hybrid Cohort Option
The on-campus/hybrid cohort option is designed for the student that works best in a team setting alongside other students with daily faculty interaction. On-campus/Hybrid students will participate in didactic lectures interacting in real time with faculty and other students. With three starting points each year (Summer, Fall & Spring) students in this program can begin classes at the time most convenient for them. The average time to complete the program is eighteen months with full-time enrollment. Visit our How to Apply website for application requirements, deadlines, and other details.
Online Track Option
The online option is designed for the student balancing work and family responsibilities with enrollment. The MS degree is completed online except for an internship practicum experience. Due to the complexity of completing an internship practicum experience in a foreign location or a remote location other than DFW, students enrolled in the online track option will have their internship practicum replaced with a capstone project worth 6 SCH. In addition to the rigorous curriculum, students will have online access to advising. With three starting points each year, students in this program can begin classes at the time most convenient for them. The average time to complete the program is eighteen months with full-time enrollment. Visit our How to Apply website for application requirements, deadlines, and other details.
Campus resources are available from Financial Aid to the Library, including Career Services and the Center for Academic Performance, without traveling to our physical location.
What can I do with a graduate degree in Clinical Research Management?
Well-trained clinical research professionals are in high demand. The tremendous increase in medical technology and information in the last decade has resulted in an explosion of potential new drugs, devices and biologics that must be tested before being released for use by the public. The profession is constantly challenged to improve and streamline the clinical research programs in order to shorten the development timelines and control the cost for new product development.
Clinical research professionals can hold a multitude of positions either in industry, at the investigational site, or in the clinical research service profession either at a contract research organization (CRO) or a site management organization (SMO). Job titles may include, but are not restricted to, Clinical Research Associate (CRA), Clinical Research Scientist (CRS), Clinical Research Coordinator (CRC), Medical Writer, Clinical Trial Auditor, Clinical Trial Monitor, Product Safety Specialist, Clinical Research Trainer, etc. Industry (sponsor) and service professions (CRO, SMO) usually provide technical and managerial career paths and ample growth opportunities.
Typically a clinical research coordinator who has been involved with the implementation and coordination of a clinical trial at a research site (private, clinic, hospital), will advance his/her career by switching to either industry or one of the service professions. Others make the reverse switch because they prefer the interactions with the patients, or they may want to travel less than what is typically required from a clinical trial monitor. Turnover in all these industries and positions is relatively high because of the growing variety of choices clinical research professionals have, especially after they have accumulated a number of years of experience.
What are the program requirements?
Each student is responsible for the completion of the requirements for the Clinical Research Management program according to the procedures that follow. Each item must be completed in the sequence and time period indicated. Forms are subject to revision at any time and can be obtained from the SBS Forms and Guidelines site.
- The admissions committee will review all applicants for acceptance into the MS program in Clinical Research Management. A student must have a bachelor’s degree and must meet the general admission requirements as described in the catalog in effect at the time of application.
- The student will be assigned a major professor and an advisory committee consisting of the major professor and an advisory committee consisting of the mentor and two other graduate faculty members. The names of these individuals will be filed on the designation of advisory committee form with the SBS Office of Student Services. A degree plan must also be filed at this time.
- After the coursework has been completed, the student will enroll in Internship Practicum (BMSC 5697) or a Capstone project. The student will complete a six-month unpaid internship at a site previously approved by the graduate school. The student is responsible for transportation to and from the site. During this time, the student will learn how to perform the duties expected of particular clinical research positions in clinical research centers such as a hospital or clinic, pharmaceutical or medical device company, a clinical research organization or site management organization. The student must be in good academic standing prior to beginning the Internship Practicum. Exceptions to this rule can only be granted by the dean or his designee. Online track students will have the option of completing a capstone project in lieu of the internship practicum.
- A formal research proposal describing how the practicum is to be spent must be approved by the advisory committee and submitted to the graduate school.
- At the conclusion of the practicum/capstone project, the student will present his/her work as both oral and written reports. The oral presentation will be open to the public and will then be followed by a private defense with the advisory committee. The student must submit a first draft of the practicum report/capstone project and internship daily journal to the major professor for review prior to the public seminar. The major professor must approve the internship practicum report/capstone project report before it is distributed to the committee members by the student. The final written report should be given to the committee no later than two weeks before the formal defense. Students should coordinate the reservation of a seminar room with the Graduate School office at least one month prior to the defense. At this time the committee will either approve/or not approve the work of the internship and the report. If not approved, the student may have a chance to revise the report or repeat the practicum one time at the discretion of the committee. The major professor, together with the other members of the committee, will assign a letter grade to the practicum/capstone project. The report must be submitted in accordance with the instructions for completing graduation requirements within the deadlines for graduation published in the academic calendar. A more detailed description of the internship practicum/capstone project and report requirements may be found in the Internship Practicum Guidelines available on the SBS Graduation website.
- It is strongly suggested that the student and major professor, as well as the major professor and the on-site mentor, communicate on a regular basis to review the student’s progress during the practicum/capstone project.
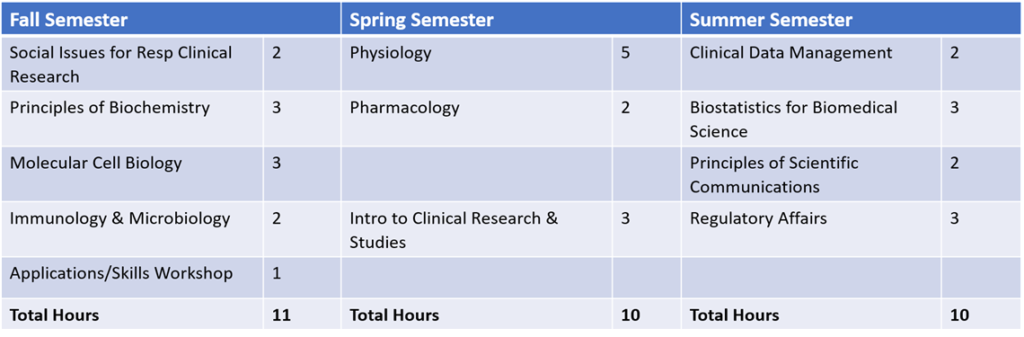
Curriculum
The graphic below details the curriculum requirements.
Internship Practicum (BMSC 5697) 18 SCH with practicum report/defense
Total Semester Credit Hours = 49
Online Track Capstone Project 6 SCH with project report/defense
Total Semester Credit Hours for Online Track = 37
Internship Practicum (BMSC 5697)
The Internship Practicum provides a hands-on training experience for the graduate student whose master’s degree will be in the specialized discipline of Clinical Research Management. The program director will identify approved, on-sight and off-campus internship opportunities in north Texas and will work to place students at suitable sites. The internship may take place either on-campus or at an approved off-campus site in the Dallas-Fort Worth-Denton Metroplex and, in some cases, at a site in other parts of the state or country. UNTHSC does not offer any remuneration to the student when he/she is enrolled in BMSC 5697 and the student should not expect to be paid as an intern. Students will be expected to provide for their own transportation and housing needs during the internship experience. The student is expected to keep a daily journal during this experience. At the end of the practicum, the student will write a report detailing the activities of the internship. The student’s advisory committee must approve this report together with the daily journal. The student must make a formal presentation to the advisory committee and defend the work at this time. A copy of the report must be submitted within the appropriate deadlines for graduation as published in the SBS Academic Calendar.
Capstone project (online track students only)
The capstone project is a required component of the online Clinical Research Management Master’s program that will replace the requirement of an internship practicum. The students will enroll for 3 SCH each in two semesters for a total of 6 SCH for their capstone project. The purpose of the capstone project is to equip students with the knowledge and skills required to contribute to the field of clinical research management. This individualized scholarly work may consist of a detailed case study, literature review, and data analysis project or a clinical research project, or a clinical quality improvement project. Students will be paired with a mentor from UNTHSC or our partner healthcare organizations to oversee their work. At the beginning of the capstone, the mentor and student will identify a topic or a specific problem to address or investigate. They will then construct an action plan or research proposal and the student will conduct the data analysis/literature review. At the end of the project, the student will complete a project report and do an oral presentation of the project.


Social media